Drosomycin
Drosomycin is an antifungal peptide from Drosophila melanogaster and was the first antifungal peptide isolated from insects.[1] Drosomycin is induced by infection by the Toll signalling pathway,[2] while expression in surface epithelia like the respiratory tract is instead controlled by the immune deficiency pathway (Imd).[3] This means that drosomycin, alongside other antimicrobial peptides (AMPs) such as cecropins,[4][5] diptericin,[6] drosocin,[7] metchnikowin[8] and attacin[9], serves as a first line defence upon septic injury. However drosomycin is also expressed constitutively to a lesser extent in different tissues and throughout development.[10]
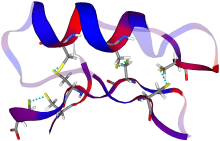
Structure
Drosomycin is a 44-residue defensin-like peptide containing four disulfide bridges. These bridges stabilize a structure involving one α-helix and three β-sheets. Owing to these four disulfide bridges, drosomycin is resistant to degradation and the action of proteases.[11][12][13] The cysteine stabilized αβ motif of drosomycin is also found in Drosophila defensin, and some plant defensins. Drosomycin has greater sequence similarity with these plant defensins (up to 40%), than with other insect defensins.[14] The structure was discovered in 1997 by Landon and his colleagues[15] The αβ motif of drosomycin is also found in a scorpion neurotoxin, and drosomycin potentiates the action of this neurotoxin on nerve excitation.[16]
Drosomycin multigene family
At the nucleotide level, drosomycin is a 387 bp-long gene (Drs) which lies on Müller element 3L,[17] very near six other drosomycin-like (Drsl) genes. These various drosomycins are referred to as the drosomycin multigene family. However only drosomycin itself is part of the systemic immune response, while the other genes are regulated in different fashions. The antimicrobial activity of these various drosomycin-like peptides also differs.[18][19] In 2015 Gao and Zhu[20] found that in some Drosophila species (D. takahashii) some of these genes have been duplicated and this Drosophila has 11 genes in the drosomycin multigene family in total.
Function
It seems that drosomycin has about three major functions on fungi, the first is partial lysis of hyphae, the second is inhibition of spore germination (in higher concentrations of drosomycin), and the last is delaying of hypha growth, which leads to hyphae branching (at lower concentrations of drosomycin).[21] The exact mechanism of function to fungi still has to be clarified. In 2019, Hanson and colleagues[22] generated the first drosomycin mutant, finding that indeed flies lacking drosomycin were more susceptible to fungal infection.
References
- Fehlbaum P, Bulet P, Michaut L, Lagueux M, Broekaert WF, Hetru C, Hoffmann JA (December 1994). "Insect immunity. Septic injury of Drosophila induces the synthesis of a potent antifungal peptide with sequence homology to plant antifungal peptides". The Journal of Biological Chemistry. 269 (52): 33159–63. PMID 7806546.
- Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA (September 1996). "The dorsoventral regulatory gene cassette spätzle/Toll/cactus controls the potent antifungal response in Drosophila adults". Cell. 86 (6): 973–83. doi:10.1016/S0092-8674(00)80172-5. PMID 8808632.
- Zhang ZT, Zhu SY (October 2009). "Drosomycin, an essential component of antifungal defence in Drosophila". Insect Molecular Biology. 18 (5): 549–56. doi:10.1111/j.1365-2583.2009.00907.x. PMID 19754735.
- Kylsten P, Samakovlis C, Hultmark D (January 1990). "The cecropin locus in Drosophila; a compact gene cluster involved in the response to infection". The EMBO Journal. 9 (1): 217–24. doi:10.1002/j.1460-2075.1990.tb08098.x. PMC 551649. PMID 2104802.
- Tryselius Y, Samakovlis C, Kimbrell DA, Hultmark D (February 1992). "CecC, a cecropin gene expressed during metamorphosis in Drosophila pupae". European Journal of Biochemistry. 204 (1): 395–9. doi:10.1111/j.1432-1033.1992.tb16648.x. PMID 1740152.
- Wicker C, Reichhart JM, Hoffmann D, Hultmark D, Samakovlis C, Hoffmann JA (December 1990). "Insect immunity. Characterization of a Drosophila cDNA encoding a novel member of the diptericin family of immune peptides". The Journal of Biological Chemistry. 265 (36): 22493–8. PMID 2125051.
- Bulet P, Dimarcq JL, Hetru C, Lagueux M, Charlet M, Hegy G, et al. (July 1993). "A novel inducible antibacterial peptide of Drosophila carries an O-glycosylated substitution". The Journal of Biological Chemistry. 268 (20): 14893–7. PMID 8325867.
- Levashina EA, Ohresser S, Bulet P, Reichhart JM, Hetru C, Hoffmann JA (October 1995). "Metchnikowin, a novel immune-inducible proline-rich peptide from Drosophila with antibacterial and antifungal properties". European Journal of Biochemistry. 233 (2): 694–700. doi:10.1111/j.1432-1033.1995.694_2.x. PMID 7588819.
- Asling B, Dushay MS, Hultmark D (April 1995). "Identification of early genes in the Drosophila immune response by PCR-based differential display: the Attacin A gene and the evolution of attacin-like proteins". Insect Biochemistry and Molecular Biology. 25 (4): 511–8. doi:10.1016/0965-1748(94)00091-C. PMID 7742836.
- Ferrandon D, Jung AC, Criqui M, Lemaitre B, Uttenweiler-Joseph S, Michaut L, et al. (August 1998). "A drosomycin-GFP reporter transgene reveals a local immune response in Drosophila that is not dependent on the Toll pathway". The EMBO Journal. 17 (5): 1217–27. doi:10.1093/emboj/17.5.1217. PMC 1170470. PMID 9482719.
- P. Fehlbaum, P. Bulet, L. Michaut, M. Lagueux, W.F. Broekaert, C. Hetru, J.A. Hoffmann. Insect Immunity: septic injury of Drosophila induces the synthesis of a potent antifungal peptide with sequence homology to plant antifungal peptides. Journal of Biological Chemistry, 269 (1994), pp. 33159–33163
- L. Michaut, P. Fehlbaum, M. Moniatte, A. Van Dorsselaer, J.M. Reichhart, P. Bulet. Determination of the disulfide array of the first inducible antifungal peptide from insects: drosomycin from Drosophila melanogaster. FEBS Letters, 395 (1996), pp. 6–10
- S. Uttenweiler-Joseph, M. Moniatte, M. Lagueux, A. Van Dorsselaer, J.A. Hoffmann, P. Bulet. Differential display of peptides induced during the immune response of Drosophila: a matrix-assisted laser desorption ionization time-of-flight mass spectrometry study. Proceedings of the National Academy of Sciences USA, 95 (1998), pp. 11342–11347
- Fant F, Vranken W, Broekaert W, Borremans F (May 1998). "Determination of the three-dimensional solution structure of Raphanus sativus antifungal protein 1 by 1H NMR". Journal of Molecular Biology. 279 (1): 257–70. doi:10.1006/jmbi.1998.1767. PMID 9636715.
- Landon C, Sodano P, Hetru C, Hoffmann J, Ptak M (September 1997). "Solution structure of drosomycin, the first inducible antifungal protein from insects". Protein Science. 6 (9): 1878–84. doi:10.1002/pro.5560060908. PMC 2143780. PMID 9300487.
- Cohen L, Moran Y, Sharon A, Segal D, Gordon D, Gurevitz M (August 2009). "Drosomycin, an innate immunity peptide of Drosophila melanogaster, interacts with the fly voltage-gated sodium channel". The Journal of Biological Chemistry. 284 (35): 23558–63. doi:10.1074/jbc.M109.023358. PMC 2749130. PMID 19574227.
- "Drs Drosomycin [Drosophila melanogaster (fruit fly)] – Gene – NCBI". www.ncbi.nlm.nih.gov. Retrieved 2017-01-04.
- F. M. Jiggins and K. W. Kim, “The evolution of antifungal peptides in Drosophila,” Genetics, vol. 171, no. 4, pp. 1847–1859, 2005
- W. Y. Yang, S. Y. Wen, Y. D. Huang, et al., “Functional divergence of six isoforms of antifungal peptide Drosomycin in Drosophila melanogaster,” Gene, vol. 379, pp. 26–32, 2006
- Gao B, Zhu S (August 2016). "The drosomycin multigene family: three-disulfide variants from Drosophila takahashii possess antibacterial activity". Scientific Reports. 6: 32175. Bibcode:2016NatSR...632175G. doi:10.1038/srep32175. PMC 4999892. PMID 27562645.
- Bulet P, Hetru C, Dimarcq JL, Hoffmann D (1999-06-01). "Antimicrobial peptides in insects; structure and function". Developmental and Comparative Immunology. 23 (4–5): 329–44. doi:10.1016/S0145-305X(99)00015-4. PMID 10426426.
- Hanson MA, Dostálová A, Ceroni C, Poidevin M, Kondo S, Lemaitre B (February 2019). "Synergy and remarkable specificity of antimicrobial peptides in vivo using a systematic knockout approach". eLife. 8. doi:10.7554/eLife.44341. PMC 6398976. PMID 30803481.