Donor (semiconductors)
In semiconductor physics, a donor is a dopant atom that, when added to a semiconductor, can form a n-type region.
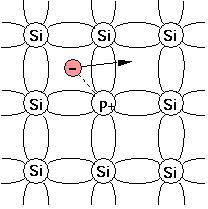
For example, when silicon (Si), having four valence electrons, needs to be doped as a n-type semiconductor, elements from group V like phosphorus (P) or arsenic (As) can be used because they have five valence electrons. A dopant with five valence electrons is also called a pentavalent impurity. [1] Other pentavalent dopants are antimony (Sb) and bismuth (Bi).
When substituting a Si atom in the crystal lattice, four of the valence electrons of phosphorus form covalent bonds with the neighbouring Si atoms but the fifth one remains weakly bonded. The initially electro-neutral donor becomes positively charged (ionised). At room temperature, the fifth electron is liberated, can move around the Si crystal and carry a current, and thus act as a charge carrier.
See also
References
- "Fundamentals: Doping: n- and p-semiconductors". www.halbleiter.org. Retrieved 2016-12-19.