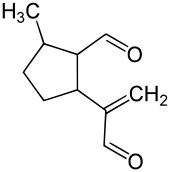
Dolichodial
Dolichodial is a natural chemical compound with two aldehyde groups, which belongs to the group of iridoids.
 | |
| Names | |
|---|---|
| IUPAC name
2-Methyl-5-(3-oxo-1-propen-2-yl)cyclopentanecarbaldehyde | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
| |
| Properties | |
| C10H14O2 | |
| Molar mass | 166.220 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Chemistry
It has in its five-membered ring three asymmetric carbon atoms and accordingly exists in four diastereomeric pairs of enantiomers. The pairs with a different stereochemistry of dolichodial are called anisomorphal and peruphasmal.
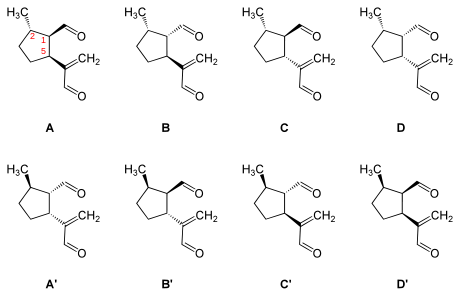
- (1R,2S,5S) = (−)-Dolichodial (A)
- (1S,2S,5S) = (+)-Anisomorphal (B)
- (1R,2S,5R) = Peruphasmal (C)
- (1S,2S,5R) = D
- (1S,2R,5R) = (+)-Dolichodial (A’)
- (1R,2R,5R) = (−)-Anisomorphal (B’)
- (1S,2R,5S) = Peruphasmal (C’)
- (1R,2R,5S) = D’
Occurrence
Dolichodial and its stereoisomers can be found in the essential oils of certain plants, and also in the defensive secretions of some insect species.[1][2][3]
gollark: Yes, gaming can occur.
gollark: No.
gollark: GTech™ Apiary Site-101.
gollark: LyricLy doesn't though.
gollark: Hi, I exist now.
References
- Tschuch G, Lindemann P, Moritz G (2008). "An unexpected mixture of substances in the defensive secretion of the Tubuliferan thrips, Callococcus fuscipennis". Journal of Chemical Ecology. 34: 742–747. doi:10.1007/s10886-008-9494-3.
- Boevé JL, Braekman JC, Daloze D, Houart M, Pasteels JM (1984). "Defensive secretions of Nematinae larvae (Symphyta - Tenthredinidae)". Cellular and Molecular Life Sciences. 40: 546–547. doi:10.1007/BF01982322.
- Dossey AT, Walse S, Edison AS (2008). "Developmental and geographical variation in the chemical defense of the walkingstick insect Anisomorpha buprestoides". Journal of Chemical Ecology. 34 (5): 584–590. doi:10.1007/s10886-008-9457-8. PMID 18401661.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.