Dodecacalcium hepta-aluminate
Dodecacalcium hepta-aluminate (12CaO·7Al2O3, Ca12Al14O33 or C12A7) is an inorganic solid that occurs rarely in nature as the mineral mayenite. It is an important phase in calcium aluminate cements and is an intermediate in the manufacture of Portland cement. Its composition and properties have been the subject of much debate, because of variations in composition that can arise during its high-temperature formation.[5]
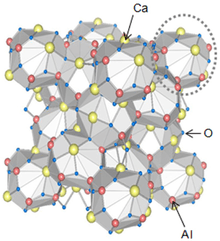 Crystal structure of C12A7[1] | |
| Names | |
|---|---|
| Other names
C12A7; mayenite; Tetradecaaluminum dodecacalcium tritriacontaoxide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| Ca12Al14O33 | |
| Molar mass | 1,386.66 g·mol−1 |
| Appearance | Clear to black solid, depending on synthesis and doping[2] |
| Density | 2.68 g·cm−3 |
| Melting point | 1,400 °C (2,550 °F; 1,670 K) |
Refractive index (nD) |
1.614–1.643[3] |
| Structure | |
| Cubic | |
| I43d | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis
Polycrystalline C12A7 can be prepared via a conventional solid-state reaction, i.e., heating a mixture of calcium carbonate and aluminium oxide or aluminium hydroxide powders, in air. It is not formed in oxygen or in moisture-free atmosphere. It can be regrown into single crystals using the Czochralski or zone melting techniques.[2]
In Portland cement kilns, C12A7 is an early reaction product of aluminium and calcium oxides in the temperature range 900–1200 °C. With the onset of melt-phases at higher temperatures, it reacts with further calcium oxide to form tricalcium aluminate. It thus can appear in under-burned kiln products. It also occurs in some natural cements.
Composition and structure
The mineral as normally encountered is a solid solution series with end-members Ca12Al14O33 and Ca6Al7O16(OH). The latter composition loses water only at high temperature, and has lost most of it by the melting point (around 1400 °C). If material heated to this temperature is rapidly cooled to room temperature, the anhydrous composition is obtained. The rate of re-absorption of water to form the hydrous composition is negligible below 930 °C.
C12A7 has a cubic crystal symmetry; Ca12Al14O33 has a lattice constant of 1.1989 nm[4] and a density of 2.680 g·cm−3 while Ca6Al7O16(OH) has 1.1976 nm and 2.716 g·cm−3. The unit cell consists of 12 cages with the inner diameter of 0.44 nm and a formal charge of +1/3, two of them host free O2− ions (not shown in the infobox structure). These ions can easily move through the material and can be replaced by F−, Cl− (as in the mineral chlormayenite) or OH− ions.[2]
The confusion regarding composition contributed to the mistaken assignment of the composition Ca5Al3O33. Studies of the system have shown that the solid solution series extends also to the accommodation of other species in place of the hydroxyl group, including halides, sulfide and oxide ions.[5]
Properties and applications
C12A7 is an important mineral phase in calcium aluminate cements[1] and is an intermediate in the manufacture of Portland cement. It reacts rapidly with water, with considerable heat evolution, to form 3CaO·Al2O3·6H2O and Al(OH)3 gel. The formation of the hydrate from this mineral and from monocalcium aluminate represents the first stage of strength development in aluminous cements. Because of its higher reactivity, leading to excessively rapid hydration, aluminous cements contain relatively low amounts of dodecacalcium hepta-aluminate, or none at all.
C12A7 has potential applications in optical, bio and structural ceramics. Some amorphous calcium aluminates are photosensitive and hence are candidates for optical information storage devices.[6][7][8] They also have desirable infrared transmission properties for optical fibers.[9][10]
While undoped C12A7 is a wide-bandgap insulator, electron-doped electride C12A7:e− is a metallic conductor with a conductivity reaching 1500 S/cm at room temperature; it may even exhibit superconductivity upon cooling to 0.2–0.4 K. C12A7:e− is also a catalyst that has potential applications in the ambient-pressure synthesis of ammonia. Electron doping is achieved by extracting O2− ions from the C12A7 structure via chemical reduction. The injected electrons occupy a unique conduction band called 'the cage conduction band', and migrate through the C12A7:e− crystal by tunneling. They can be readily and reversibly replaced with hydride ions (H−) by heating C12A7:e− in a hydrogen atmosphere. Owing to this reversibility, C12A7:e− does not suffer from hydrogen poisoning – irreversible deterioration of properties upon exposure to hydrogen which is common to traditional catalysts used in the ammonia synthesis.[1][2]
References
- Hosono, H.; Tanabe, K.; Takayama-Muromachi, E.; Kageyama, H.; Yamanaka, S.; Kumakura, H.; Nohara, M.; Hiramatsu, H.; Fujitsu, S. (2015). "Exploration of new superconductors and functional materials, and fabrication of superconducting tapes and wires of iron pnictides". Science and Technology of Advanced Materials. 16 (3): 033503. arXiv:1505.02240. Bibcode:2015STAdM..16c3503H. doi:10.1088/1468-6996/16/3/033503. PMC 5099821. PMID 27877784.
- Ginley, David; Hosono, Hideo; Paine, David C. (2010). Handbook of Transparent Conductors. Springer Science & Business Media. pp. 318 ff. ISBN 978-1-4419-1638-9.
- Anthony, John W.; Bideaux, Richard A.; Bladh, Kenneth W.; Nichols, Monte C., eds. (1997). "Mayenite". Handbook of Mineralogy (PDF). III (Halides, Hydroxides, Oxides). Chantilly, VA, US: Mineralogical Society of America. ISBN 0962209724.
- Grandfield, John (2014). Light Metals 2014. Wiley. p. 78. ISBN 978-1-118-88840-7.
- Taylor, H F W (1990) Cement Chemistry, Academic Press, ISBN 0-12-683900-X, pp. 36–38
- Gulgun, M. A.; Popoola, O. O.; Kriven, W. M. (1994). "Chemical Synthesis and Characterization of Calcium Aluminate Powders". Journal of the American Ceramic Society. 77 (2): 531. doi:10.1111/j.1151-2916.1994.tb07026.x.
- Wallenberger, F. T.; Weston, N. E.; Brown, S. D. (1991). "Melt-processed calcium aluminate fibers: Structural and optical properties". Growth and Characterization of Materials for Infrared Detectors and Nonlinear Optical Switches. Growth and Characterization of Materials for Infrared Detectors and Nonlinear Optical Switches. 1484. p. 116. doi:10.1117/12.46516.
- Birchall, J. D.; Howard, A. J.; Kendall, K. (1981). "Flexural strength and porosity of cements". Nature. 289 (5796): 388. Bibcode:1981Natur.289..388B. doi:10.1038/289388a0.
- Kendall, K.; Howard, A. J.; Birchall, J. D.; Pratt, P. L.; Proctor, B. A.; Jefferis, S. A. (1983). "The Relation between Porosity, Microstructure and Strength, and the Approach to Advanced Cement-Based Materials [and Discussion]". Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences. 310 (1511): 139. Bibcode:1983RSPTA.310..139K. doi:10.1098/rsta.1983.0073.
- Goktas, A. A.; Weinberg, M. C. (1991). "Preparation and Crystallization of Sol-Gel Calcia-Alumina Compositions". Journal of the American Ceramic Society. 74 (5): 1066. doi:10.1111/j.1151-2916.1991.tb04344.x.