Dithioerythritol
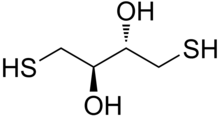
Dithioerythritol (DTE) is a sulfur containing sugar derived from the corresponding 4-carbon monosaccharide erythrose. It is an epimer of dithiothreitol (DTT). The molecular formula for DTE is C4H10O2S2.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
(2R,3S)-1,4-Bis(sulfanyl)butane-2,3-diol | |
| Other names
(2R,3S)-1,4-Dimercaptobutane-2,3-diol (no longer recommended) 2,3-Dihydroxybutane-1,4-dithiol Erythro-2,3-dihydroxy-1,4-butanedithiol Erythro-1,4-dimercapto-2,3-butanediol Cleland's reagent (also used for DTT) | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.027.271 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H10O2S2 | |
| Molar mass | 154.253 g/mol |
| Melting point | 82 to 84 °C (180 to 183 °F; 355 to 357 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Like DTT, DTE makes an excellent reducing agent, although its standard reduction potential is not quite as negative, i.e., DTE is slightly less effective at reducing than DTT. This is presumably because the orientation of the OH groups in its cyclic disulfide-bonded form (oxidized form) is less stable due to greater steric repulsion than their orientation in the disulfide-bonded form of DTT. In the disulfide-bonded form of DTT, these hydroxyl groups are trans to each other, whereas they are cis to each other in DTE.
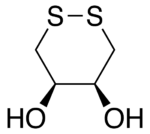
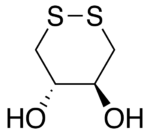
The oxidized forms of dithioerythritol (DTE, left) and dithiothreitol (DTT, right).
References
- Cleland, W.W. (April 1964). "Dithiothreitol, A New Protective Reagent for SH Groups". Biochemistry. 3 (4): 480–2. doi:10.1021/bi00892a002. PMID 14192894.
External links

This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.