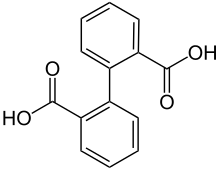
Diphenic acid
Diphenic acid is an organic compound with the formula (C6H4CO2H)2. It is the most studied of several isomeric dicarboxylic acids of biphenyl. It is a white solid that can be prepared in the laboratory from anthranilic acid via the diazonium salt.[1] It is the product of the microbial action on phenanthrene.[2]
 | |
| Names | |
|---|---|
| Other names
2,2'-dibenzoic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.006.889 |
| EC Number |
|
| 536420 | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C14H10O4 | |
| Molar mass | 242.230 g·mol−1 |
| Appearance | white solid |
| Density | 1.2917 g/cm3 |
| Melting point | 235.5 °C (455.9 °F; 508.6 K) |
| Hazards | |
| GHS pictograms |  |
| GHS Signal word | Warning |
GHS hazard statements |
H315, H319, H335 |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The compound forms a variety of coordination polymers.[3] It also exhibits atropisomerism.
References
- Atkinson, E. R.; Lawler, H. J. (1927). "Diphenic Acid". Organic Syntheses. 7: 30. doi:10.15227/orgsyn.007.0030.
- Moody, J. D.; Freeman, J. P.; Doerge, D. R.; Cerniglia, C. E. (2001). "Degradation of Phenanthrene and Anthracene by Cell Suspensions of Mycobacterium sp. Strain PYR-1". Applied and Environmental Microbiology. 67 (4): 1476–1483. doi:10.1128/AEM.67.4.1476-1483.2001. PMC 92757. PMID 11282593.
- Yang, Jin; Ma, Jian-Fang; Liu, Ying-Ying; Ma, Ji-Cheng; Batten, Stuart R. (2009). "A Series of Lead(II) Complexes with π−π Stackings: Structural Diversities by Varying the Ligands". Crystal Growth & Design. 9 (4): 1894–1911. doi:10.1021/cg801085d.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.