Dioecy
Dioecy (Greek: διοικία "two households"; adjective form: dioecious) is a characteristic of a species, meaning that it has distinct male and female individual organisms.[1] Dioecious reproduction is biparental reproduction. Dioecy is one method that excludes self-fertilization and promotes allogamy (outcrossing), and thus tends to reduce the expression of recessive deleterious mutations present in a population.[2] Flowering plants have several other methods of excluding self-fertilization, called self-incompatibility.
In zoology

In zoology, dioecious species may be opposed to hermaphroditic species, meaning that an individual is of only one sex, in which case the synonym gonochory is more often used. Dioecy may also describe colonies within a species, such as the colonies of Siphonophorae (Portuguese man-of-war), which may be either dioecious or monoecious.[4] An individual dioecious colony contains members of only one sex, whereas monoecious colonies contain members of both sexes.
Most animal species are dioecious (gonochoric).[5]
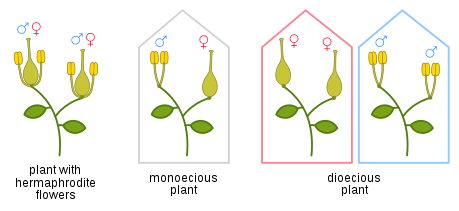
In botany
Dioecious species have the male and female reproductive structures on separate plants.[6][7] The meaning of male and female in the context of plants is different from that used in animal groups and the usage is not strictly correct. This issue is discussed below in the Alternation of generations section. About 6 percent of angiosperm species are entirely dioecious and about 7% of angiosperm genera contain some dioecious species.[8] Examples of dioecious plant species include ginkgos, willows, cannabis and African teak. More examples of dioecious plants can be found in the Wikipedia category, Dioecious plants.
Dioecy occurs in a wide variety of plant families. However it is more common in woody plants[9] and heterotrophic species.[10] Certain algae are dioecious.[11]
Alternatives to dioecy
There are several alternatives to dioecy for sexual reproductive structure organization in plants including bisexual flowers, monoecy, gynomonoecy, andromonoecy. These are described at Plant reproductive morphology.
Alternation of generations
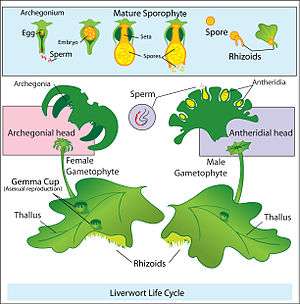
Land plants have alternation of generations, for which the sporophyte generation produces spores rather than gametes. Strictly speaking, the sporophytes of land plants do not have either male or female reproductive organs. The gametophytes of flowering plants are of a single sex; the male gametophytes are contained within the pollen, and the female gametophytes are contained within ovules. The sporophyte generation is called dioecious when each sporophyte has only one kind of spore-producing organ whose spores ultimately give rise to either all male gametes (sperm) or all female gametes (eggs). Slightly different terms, "dioicous" and "monoicous", may be used for the gametophyte generation, although "dioecious" and "monoecious" are also used.[12]
In mycology
Monoecy and dioecy in fungi refer to the donor and recipient roles in mating, where a nucleus is transferred from one haploid hypha to another, and the two nuclei then present in the same cell merge by karyogamy to form a zygote.[13] The definition avoids reference to male and female reproductive structures, which are rare in fungi.[13] An individual of a dioecious fungal species not only requires a partner for mating, but performs only one of the roles in nuclear transfer, as either the donor or the recipient. A monoecious fungal species can perform both roles, but may not be self-compatible.[13]
Adaptive benefit
Dioecy has a demographic disadvantage compared with hermaphroditism: only about half of reproductive adults produce seeds. It is commonly assumed that dioecious species must therefore have fitness advantages to compensate for this cost through increased survival, growth, or reproduction. In trees, compensation is realized mainly through increased seed production by females. This in turn is facilitated by a lower contribution of reproduction to population growth, which results in no demonstrable net costs of having males in the population compared to being hermaphroditic.[14] Dioecy may also accelerate or retard lineage diversification in angiosperms. Dioecious lineages are more diversified in certain genera, but less in others. An analysis suggested that dioecy neither consistently places a strong brake on diversification, nor strongly drives it.[15]
See also
- Gonochorism
- Hermaphrodite
- Plant reproductive morphology
- Self-incompatibility in plants
- Sexual dimorphism
References
- K. S. Bawa (1980). "Evolution of Dioecy in Flowering Plants". Annual Review of Ecology and Systematics. 11: 15–39. doi:10.1146/annurev.es.11.110180.000311. JSTOR 2096901.
- Charlesworth D, Willis JH (2009). "The genetics of inbreeding depression". Nat. Rev. Genet. 10 (11): 783–96. doi:10.1038/nrg2664. PMID 19834483.
- "Animal Diversity Web". Retrieved 27 April 2014.
- Dunn, C.W.; Pugh, P.R.; Haddock, S.H.D. (2005). "Molecular Phylogenetics of the Siphonophora (Cnidaria), with Implications for the Evolution of Functional Specialization". Systematic Biology. 54 (6): 916–935. doi:10.1080/10635150500354837. PMID 16338764.
- David, J.R. (2001). "Evolution and development: some insights from evolutionary theory". Anais da Academia Brasileira de Ciências. 73 (3): 385–395. doi:10.1590/s0001-37652001000300008.
- "dioecious - Kew glossary". Archived from the original on 5 May 2014. Retrieved 4 May 2014.
- Hickey, M. & King, C. (2001). The Cambridge Illustrated Glossary of Botanical Terms. Cambridge University Press.
- Renner, S. S.; R. E. Ricklefs (1995). "Dioecy and its correlates in the flowering plants". American Journal of Botany. 82 (5): 596–606. doi:10.2307/2445418. JSTOR 2445418.
- Matallana, G.; Wendt, T.; Araujo, D.S.D.; Scarano, F.R. (2005), "High abundance of dioecious plants in a tropical coastal vegetation", American Journal of Botany, 92 (9): 1513–1519, doi:10.3732/ajb.92.9.1513, PMID 21646169CS1 maint: uses authors parameter (link)
- Nickrent D.L., Musselman L.J. (2004). "Introduction to Parasitic Flowering Plants". The Plant Health Instructor. doi:10.1094/PHI-I-2004-0330-01. Archived from the original on 2016-10-05. Retrieved 2017-01-10.
- Maggs, C.A. and Hommersand, M.H. 1993. Seaweeds of the British Isles Volume 1 Rhodophyta Part 3A Ceramiales. The Natural History Museum, London. ISBN 0-11-310045-0
- Stearn, W.T. (1992). Botanical Latin: History, grammar, syntax, terminology and vocabulary, Fourth edition. David and Charles.
- Esser, K. (1971). "Breeding systems in fungi and their significance for genetic recombination". Molecular and General Genetics MGG. 110 (1): 86–100. doi:10.1007/bf00276051.
- Bruijning, Marjolein; Visser, Marco D.; Muller-Landau, Helene C.; Wright, S. Joseph; Comita, Liza S.; Hubbell, Stephen P.; de Kroon, Hans; Jongejans, Eelke (2017). "Surviving in a Cosexual World: A Cost-Benefit Analysis of Dioecy in Tropical Trees". The American Naturalist. 189 (3): 297–314. doi:10.1086/690137. hdl:2066/168955. ISSN 0003-0147.
- Sabath, Niv; Goldberg, Emma E.; Glick, Lior; Einhorn, Moshe; Ashman, Tia-Lynn; Ming, Ray; Otto, Sarah P.; Vamosi, Jana C.; Mayrose, Itay (2016). "Dioecy does not consistently accelerate or slow lineage diversification across multiple genera of angiosperms". New Phytologist. 209 (3): 1290–1300. doi:10.1111/nph.13696. PMID 26467174.