Dinitrobisphenol A
3,3'-Dinitrobisphenol A is an organic compound with the formula (HO(O2N)C6H3)2C(CH3)2. It is a yellow-orange solid prepared by nitration of bisphenol A [1][1][2]
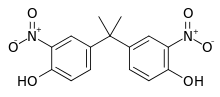 | |
| Names | |
|---|---|
| IUPAC name
2,2'-Dinitro-4,4'-(propane-2,2-di-yl)diphenol], | |
| Other names
3,3'-Dinitro-bisphenol A; 4,4'-Propane-2,2-diylbis(2-nitrophenol); Dinitro-bisphenol A; 2,2-Bis(4-hydroxy-3-nitrophenyl)propane; 4-[2-(4-Hydroxy-3-nitrophenyl)propan-2-yl]-2-nitrophenol | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| Properties | |
| C15H14N2O6 | |
| Molar mass | 318.285 g·mol−1 |
| Appearance | Yellow powder |
| Melting point | 130 °C (266 °F; 403 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Carcinogenicity
3,3'-Dinitrobisphenol A is found to be genotoxic in male ICR mice on a micronucleus test.[3] Its estrogenic potential is not known however shown some binding to estrogen-related receptor gamma to an extent.[4]
gollark: There's even JSX, where you can write HTML but in JS for some insane reason.
gollark: See, us COOL and TRENDY JS developers write ES2027 and compile it down to ES5 or so for bad browsers.
gollark: Also typescript.
gollark: > New JS is cool, but no JS developers are allowed to use them because of compatibility.Actually, we have Babel now.
gollark: That is physically impossible.
References
- Sulzberg, Theodore; Cotter, Robert J. (1969). "Synthesis and polymerization of a dinitrobisphenol a: A new polycarbonate synthesis". Journal of Polymer Science Part B: Polymer Letters. 7 (3): 185. Bibcode:1969JPoSL...7..185S. doi:10.1002/pol.1969.110070303.
- Babu, Sainath; Pathak, Chintan; Uppu, Satvika; Jones, Conrad; Fronczek, Frank R.; Uppu, Rao M. (2011). "3,3′-Dinitrobisphenol A". Acta Crystallographica Section E. 67 (10): o2556. doi:10.1107/S1600536811035458. PMC 3201564. PMID 22065402.
- Toyoizumi, Tomoyasu; Deguchi, Yuya; Masuda, Shuichi; Kinae, Naohide (2014). "Genotoxicity and Estrogenic Activity of 3,3′-Dinitrobisphenol a in Goldfish". Bioscience, Biotechnology, and Biochemistry. 72 (8): 2118. doi:10.1271/bbb.80193.
- Babu, Sainath; Vellore, Nadeem A.; Kasibotla, Agasthya V.; Dwayne, Harlan J.; Stubblefield, Michael A.; Uppu, Rao M. (2012). "Molecular docking of bisphenol a and its nitrated and chlorinated metabolites onto human estrogen-related receptor-gamma". Biochemical and Biophysical Research Communications. 426 (2): 215. doi:10.1016/j.bbrc.2012.08.065. PMID 22935422.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.