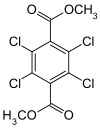
Dimethyl tetrachloroterephthalate
Dimethyl tetrachloroterephthalate (DCPA, Dacthal) is an organic compound with the formula C6Cl4(CO2CH3)2. It is a colourless solid, although commercial samples can be off white. DCPA is the dimethyl ester of tetrachloroterephthalic acid. It is used as a preemergent herbicide.[2] It kills grass and many common weeds without killing sensitive plants such as flowers, fruits, vegetables, turf, and cotton.[3] DCPA was first registered for use in the United States in 1958.[4] Production of DCPA was eventually discontinued by ISK Biosciences in 1998, but the large manufacturing company AMVAC (American Vanguard Corporation) began producing the product in 2001 for use in America.[5] In Australia, DCPA is the active ingredient in agchem company Farmalinx's herbicide called Dynamo 750.[6] Currently, DCPA is only used for eggplant, kale, turnips, and sweet potatoes.[7]
 | |
| Names | |
|---|---|
| IUPAC name
Dimethyl 2,3,5,6-tetrachlorobenzene-1,4-dicarboxylate | |
| Other names
Chlorthal-dimethyl; Dimethyl 2,3,5,6-tetrachloroterephthalate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.015.877 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H6Cl4O4 | |
| Molar mass | 331.95 g·mol−1 |
| Appearance | solid |
| Melting point | 156 °C (313 °F; 429 K) |
| 0.05 g/100 mL | |
| Vapor pressure | 0.21 mPa (2.5 x 10−6 Torr) |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | Nonflammable |
| N/A | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
>10000 mg/kg [1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Contamination
DCPA is released directly into the environment during its use as a herbicide. DCPA exists in both the vapor and particulate phases when exposed to the air. In the vapor phase, DCPA should react slowly with hydroxyl radicals with an estimated half-life of 36 days. Particulate-phase DCPA may be physically removed from air by wet and dry deposition. With a high Koc of 3900, DCPA is presumably immobile in soil, and thus may strongly attach to inorganic material in soil and other environments[7] In addition, its breakdown products, TPA (Tetrachloroterephthalic acid) and MTP (Monomethyl tetrachloroterephthalic acid), enter the environment after being formed through various processes. Studies have shown that DCPA can partially degrade through volatilization, as well as via photodegradation, but biodegradation is the primary route of DCPA degradation leading to MTP and TPA. Environmental Protection Agency testing in New York showed "measurable residues of DCPA and degradates" on land that had endured five years of treatment with DCPA, followed by three years of no treatment. DCPA is also prevalent in water and bioconcentration is seen in aquatic animals. DCPA accumulation was shown in fish at "several locations" in the United States.[7] Some of these locations included the Apalachicola, Colorado, Mobile, Savannah, and Pee Dee River Basins in both bass and carp. In the study done on these river basin locations, roughly 39% of the fish tested had stored DCPA concentrations that exceeded their limit of detection, and the male fish had higher stored concentrations of DCPA than their female counterparts.[8] Fish collected from different locations throughout the United States are often contaminated by DCPA if they are near agricultural areas that use or have used it as an herbicide.[9]
Humans are exposed to DCPA through drinking well water, eating fish, and eating leafy and root vegetables. In some areas where agriculture is prominent, inhalation of air can be a means of exposure. DCPA is listed as a Group C Possible Human Carcinogen by the National Library of Medicine.[10]
Degradation

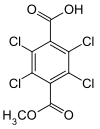
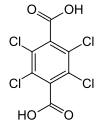
DCPA degrades via successive hydrolysis of the two ester linkages, first forming monomethyl tetrachloroterephthalate (MTP) then further to tetrachloroterephthalic acid (TPA). Following ingestion, MTP is predominantly formed in the gastrointestinal tract and TPA is formed in other tissues during the metabolism of DCPA.[11][12]
DCPA shows no chemical degradation in water that has a pH between 5.0 and 9.0. However, in the presence of sunlight, the half-life for DCPA on the surface of the water is less than three days. In soil, the half-life in the presence of sunlight ranges from 14 to 100 days.[3]
Properties of Degradates
TPA and MTP are both more water-soluble than DCPA, and readily leach into groundwater wherever DCPA is used, regardless of soil composition. TPA has been observed to cause weight loss and diarrhea in laboratory rats, the same symptoms caused by DCPA, but at lower doses than necessary for DCPA.[13] TPA does not degrade, and infiltrates soil and nearby water sources. The accumulation of TPA and its salts in areas where DCPA is widely used has prompted research on TPA, although no carcinogenicity studies have been conducted yet.[14] There have been no standard toxicity studies identified for MTP.[15]
Toxicology
Studies show that DCPA and TPA may cause detrimental health effects in laboratory animals, mainly weight loss and diarrhea occurring at doses of 2000 mg/kg/day. There were also effects on the lungs, liver, kidney, and thyroid glands of male and female rats. The LD50, or 50% lethal dose of DCPA, is 12,500 mg/kg in spartan rats, and greater than 10,000 mg/kg in beagle dogs. In humans, it seems that DCPA is poorly absorbed, as 6% of a 25-mg dose and 12% of a 50-mg dose were absorbed according to metabolites in urine. Decreased motor activity and poor sight reflexes were also observed in a study on New Zealand white rabbits that were exposed to DCPA. There were no physical developmental effects in the offspring of pregnant rats exposed to DCPA.[7] Rats whose mothers were exposed to DCPA during pregnancy had impaired higher-level learning test scores than those whose mothers were not exposed.[10] Studies regarding the carcinogenicity of DCPA have produced mixed results. A study by ISK Biotech Corp. in 1993 showed DCPA leading to thyroid tumors in male and female rats, and liver tumors in female rats. Alternatively, a 1963 study using pure DCPA did not produce any negative results when administered to albino rats.[7]
Studies have demonstrated that DCPA acts as a chemical disruptor by interfering with microtubule formation in exposed cells. This interference results in abnormal cell division. The abnormal microtubules affect cell wall formation as well as chromosome replication and division.[16] The key difference between DCPA and other mitotic inhibitors is that it often produces multinucleate cells.[17] It essentially kills plants by inhibiting cell division in this manner.[16]
Exposure to DCPA has shown damaging effects in the adrenal glands, kidneys, livers, thyroids, and spleens of laboratory animals.[16] The effects on the rabbits included decreased motor activity and poor reflexes.[18]
Regulation
United States
In the U.S. The Safe Drinking Water Act of 1996 has the U.S. EPA publish a list of contaminants referred to as the Contaminant Candidate List to assist in research efforts. The Safe Drinking Water Act also calls for the EPA to choose five contaminants from the list and determine whether regulation is necessary. In July 2008, the EPA determined that no regulatory action is necessary for DCPA mono-acid (MTP) degradate and DCPA di-acid (TPA) degradate. After multiple studies, it was determined that degradates of DCPA appear too infrequently to pose a serious health risk so the government does not regulate DCPA or its degradates in drinking water.[19] Public water systems are also not required to monitor DCPA, MTP, or TPA. There are standards set by some states ranging from 0.17 µg/L to 2 µg/L.[15]
In California, DCPA products are required to be labeled with the information that products with DCPA also contain trace amounts of Hexachlorobenzene (HCB) which is a chemical known to the State of California to cause cancer or birth defects.[20]
References
- "AMVAC MSDS No.: 291_9" (PDF). www.cdms.net. AMVAC Chemical Corporation. Archived from the original (PDF) on 2 April 2015. Retrieved 30 March 2015.
- American Vanguard Dacthal Webpage Archived 2008-11-22 at the Wayback Machine
- "Archived copy" (PDF). Archived from the original (PDF) on 2011-07-10. Retrieved 2015-03-02.CS1 maint: archived copy as title (link)
- Cox, Caroline. "DCPA (Dachtal)". Journal of Pesticide Reform. Archived from the original on 12 July 2010. Retrieved 29 March 2015.
- "Dacthal Returns to the Market; Crops, Use Patterns Remain the Same". SouthWestern Farm Press Staff. Retrieved 2015-03-22.
- Farmalinx. "DYNAMO 750 g/kg Chlorthal Dimethyl". Farmalinx. Retrieved 27 April 2015.
- "Health Effects Support for Dacthal Degredates" (PDF). ww2.epa.gov. United States Environmental Protection Agency. Retrieved 2015-02-13.
- Hinck, Jo Ellen; Schmitt, Christopher J.; Ellersieck, Mark R.; Tillitt, Donald E. (2008). "Relations between and among contaminant concentrations and biomarkers in black bass (Micropterus spp.) and common carp (Cyprinus carpio) from large U.S. rivers, 1995–2004". Journal of Environmental Monitoring. 10 (12): 1499–518. doi:10.1039/B811011E. PMID 19037492.
- Cox, Caroline. "DCPA (Dacthal)". Archived from the original on 2010-07-12. Retrieved 2015-03-18.
- "Dimethyl Tetrachloroterephthalate". Toxnet. National Library of Medicine. Retrieved 31 March 2015.
- "Dimethyl Tetrachloroterephthalate". National Library of Medicine HSDB Database. National Library of Medicine. Retrieved 12 March 2015.
- Hurto KA, Turgeon AJ, Cole MA (1979). "Degradation of Benefin and DCPA in Thatch and Soil from a Kentucky Bluegrass (Poa pratensis) Turf". Weed Soil. 27 (1979): 154–7. doi:10.1017/S0043174500043721. JSTOR 4042994.
- "Health Effects Support Document for Dacthal Degradates: Tetrachloroterephthalic Acid (TPA) and Monomethyl Tetrachloroterephthalic Acid (MTP)" (PDF). www.epa.gov. Retrieved 16 March 2015.
- Klopman, Gilles; Fercu, Dan; Rosenkranz, Herbert S. (February 1996). "The carcinogenic potential of dacthal and its metabolites". Environmental Toxicology and Chemistry. 15 (2): 80–84. doi:10.1002/etc.5620150202.
- "Summary from the Health Advisory (HA) for Dacthal and Dacthal Degradates (Tetrachloro terephthalic Acid and Monomethyl Tetrachloroterephthalic Acid)" (PDF). Health Advisory for Dacthal and Dacthal Degradates. Retrieved 31 March 2015.
- Cox, Caroline. "DCPA (Dacttal)". Archived from the original on 12 July 2010. Retrieved 18 March 2015.
- "Disruption of Mitosis" (PDF).
- "Health Effects Support Document for Dacthal Degradates: Tetrachloroterephthalic Acid (TPA) and Monomethyl Tetrachloroterephthalic Acid (MTP)" (PDF). United States Environmental Protection Agency. Retrieved 28 March 2015.
- The United States Environmental Protection Agency. "Regulatory Determination 2 for Contaminants on the Second Drinking Water Contaminant Candidate List".
- "MATERIAL SAFETY DATA SHEET" (PDF). Archived from the original (PDF) on 2 April 2015. Retrieved 8 April 2015.
