Diketopiperazine
A diketopiperazine (DKP), also known as a dioxopiperazine or piperazinedione, is a class of organic compounds related to piperazine but with two amide linkages. Three regioisomers are possible, differing in the locations of the carbonyl groups.[1]
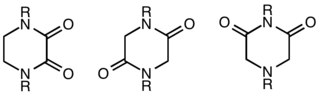
Despite their name, they are not ketones, but amides. One isomer is an oxamide obtained from ethylenediamine. 2,5-Diketopiperazines are cyclodipeptides often obtainable via condensation of two α-amino acids. 2,6-Diketopiperazines may be viewed as cyclized imide derivatives derived from iminodiacetic acids.
Of these three isomeric diketopiperazines, the 2,5-derivatives have attracted the greatest interest.[3][4] Their appearance in biologically active natural products, medicinal chemists have been inspired to use DKPs to circumvent the poor physical and metabolic properties of peptides in the course of drug discovery.
References
- Dinsmore JC, Beshore DC. (2002). "Recent Advances in the Synthesis of Diketopiperazines". Tetrahedron. 58 (17): 3297–3312. doi:10.1016/S0040-4020(02)00239-9.
- Borthwick AD, Liddle J (January 2013). "Retosiban and Epelsiban: Potent and Selective Orally available Oxytocin Antagonists". In Domling A (ed.). Methods and Principles in Medicinal Chemistry: Protein-Protein Interactions in Drug Discovery. Weinheim: Wiley-VCH. pp. 225–256. ISBN 978-3-527-33107-9.
- Borthwick, A. D. (2012). "2,5-Diketopiperazines: Synthesis, Reactions, Medicinal Chemistry, and Bioactive Natural Products",". Chem. Rev. 112: 3641-3716. doi:10.1021/cr200398y.
- Witiak DT, Wei Y. (1990). "Dioxopiperazines: chemistry and biology". Progress in Drug Research. 35: 249–363. ISBN 3-7643-2499-6. PMID 2290982.