Dihydrolevoglucosenone
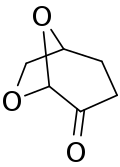
Dihydrolevoglucosenone is a bicyclic, chiral, seven-membered heterocyclic cycloalkanone which is a bio-based and fully biodegradable aprotic dipolar solvent.[3][4][5] It is a safer and "greener" alternative to toxic organic solvents such as dimethylformamide (DMF) and N-methyl-2-pyrrolidone (NMP).[6]
 | |
| Names | |
|---|---|
| IUPAC name
(1R)-7,8-Dioxabicyclo[3.2.1]octan-2-one | |
| Other names
Cyrene | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.234.612 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H8O3 | |
| Molar mass | 128.127 g·mol−1 |
| Appearance | clear to yellowish liquid |
| Density | 1.2508 g/cm3 (20 °C) [1] |
| Boiling point | 226 °C (439 °F; 499 K) [1] |
| miscible | |
| Vapor pressure | 14.4 Pa (25 °C) [1] |
Refractive index (nD) |
1.4732 (20 °C) [1] |
| Hazards[2] | |
| GHS pictograms |  |
| GHS Signal word | Warning |
GHS hazard statements |
H319 |
| P305+351+338, P313 | |
| Flash point | 108 °C (226 °F; 381 K) |
| 296 °C (565 °F; 569 K) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Presentation
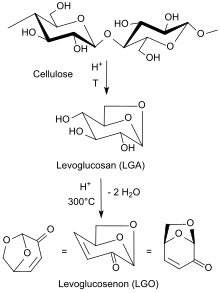
Dihydrolevoglucosenone can be prepared through the hydrogenation of unsaturated anhydrosugar levoglucosenone (LGO) by using supported metal catalysts under mild conditions. Levoglucosenone is, at the same time, a chemical building block obtained by acid-catalysed pyrolysis[7] at 300 °C of lignocellulosic biomass such as wood waste or sawdust. A significant amount of char is produced, as a by-product of LGO production, which can be used as fuel.
When cellulose in tetrahydrofuran is heated to 210 °C with low concentrations of sulfuric acid in an autoclave, up to 51% levoglucosenone can be obtained through a solvent-assisted pyrolysis.[8] Under optimized laboratory conditions, yields of up to 95% of levoglucosenone can be reached.[9]
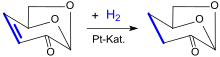
Cellulose-containing waste from biorefineries can also be converted into 6-8% LGO under microwave irradiation at 180 °C for 5 minutes, in addition to the usual decomposition products such as hydroxymethylfurfural HMF, formic acid, formaldehyde, CO2 and water.[10]
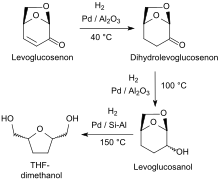
Hydrogenation of the α, β-unsaturated ketone levoglucosenone (LGO) with a metal catalyst such as palladium on alumina (Pd/Al2O3) or palladium on zirconia (Pd/ZrO2) leads selectively to Dihydrolevoglucosenone.[11][12] Further hydrogenation can also yield tetrahydrofuran-2,5-dimethanol.
Properties
Dihydrolevoglucosenone is a clear colorless, to light yellow, liquid with a comparatively high dynamic viscosity of 14.5 cP[13] (for comparison DMF: 0.92 cP at 20 °C, NMP: 1.67 cP at 25 °C) and a mild, smoky ketone-like odor.[14] It is miscible with water and many organic solvents.[14] The compound is stable at temperatures up to 195 °C and weak acids and bases. H2-LGO reacts with inorganic bases via an aldol condensation mechanism. Dihydrolevoglucosenone is readily biodegradable (99% within 14 days) and reacts to oxidants such as aqueous 30% hydrogen peroxide solution even at room temperature. Dihydrolevoglucosenone has a boiling point of 226 °C at 101.325 kPa (vs 202 °C for NMP), and a vapor pressure of 14.4 Pa near room temperature (25 °C).[1]
Applications
Dihydroglucosenone as a precursor
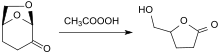
Dihydrolevoglucosenone can be used as a bio-based building block to produce a number of higher value chemicals such as drugs, flavours and fragrances and specialty polymers.[11]
The oxidation of dihydrolevoglucosenone with peracids such as peracetic acid in acetic acid produces optically pure 5-hydroxymethyldihydrofuranone [(S) - (+) - 4-hydroxymethyl-γ-butyrolactone],[15] from which zalcitabine (2'-3'-dideoxycytidine, ddC), formerly a HIV drug, is available.[16]
In a two-step hydrogenation process with a metal catalyst – first at 60 °C then at 180 °C – 1,6-hexanediol is mainly obtained via several intermediates.[17] 1,6-hexanediol can be used as a starting material for the production of polyesters, polyurethanes and diamine 1,6-diaminohexane.
At elevated temperature and in the presence of a palladium catalyst, hydrogenolysis of dihydrolevoglucosenone via levoglucosanol selectively yields tetrahydrofuran-2,5-dimethanol (THF-dimethanol),[11] which is a biodegradable solvent and a bio-based precursor to 1,6-hexanediol (and 1,6-diaminohexane).[18]
Dihydroglucosenone as a polar solvent
The search for alternative "green" solvents made from biomass or low-cost renewable raw materials, which are accessible through high-efficiency processes, in high yields, and meet the performance of conventional solvents,[19] has triggered intensive research activities in industry and academia worldwide.
Dihydrolevoglucosenone is considered a candidate as a "green" aprotic dipolar solvent.[4] Several standard reactions of organic chemistry, e.g. Menshutkin reaction,[4] Sonogashira coupling,[20] Suzuki-Miyaura coupling,[21] or the synthesis of organic ureas[22] have been carried out in dihydroglucosenone.
Production
Circa Group produces dihydrolevoglucosenone from cellulose under the Cyrene brand and has built a 50-tonne demonstration plant with partners in Tasmania. The company estimates that dihydroglucosenone performs better than NMP in 45% and comparably to NMP in 20% of trials to date. Circa received authorization in 2018 from the European Chemicals Agency (ECHA) to produce or import up to 100 tonnes per year of dihydroglucosenone to the EU.[23]
Literature
- DS van Es: Study into alternative (biobased) polar aprotic solvents. Wageningen University, Wageningen 2017 (wur.nl [PDF]).
- JH Clark, A. Hunt, C. Topi, G. Paggiola, J. Sherwood: Sustainable Solvents: Perspectives from Research, Business and Institutional Policy . Royal Society of Chemistry, London 2017, ISBN 978-1-78262-335-9 .
References
- Baird, Zachariah Steven; Uusi-Kyyny, Petri; Pokki, Juha-Pekka; Pedegert, Emilie; Alopaeus, Ville (6 Nov 2019). "Vapor Pressures, Densities, and PC-SAFT Parameters for 11 Bio-compounds". International Journal of Thermophysics. 40 (11): 102. Bibcode:2019IJT....40..102B. doi:10.1007/s10765-019-2570-9.
- Circa Group (23 January 2017). "Safety Data Sheet" (PDF).
- "Concise and Efficient Synthesis of E-stereoisomers of exo-cyclic Carbohydrate Enones. Aldol Condensation of Dihydrolevoglucosenone with Five-membered Aromatic Aldehydes1 Part 1. - PubAg". pubag.nal.usda.gov. US: United States National Agricultural Library, USA.gov. Retrieved 2019-01-31.
- Sherwood, James; De bruyn, Mario; Constantinou, Andri; Moity, Laurianne; McElroy, C. Rob; Farmer, Thomas J.; Duncan, Tony; Raverty, Warwick; Hunt, Andrew J. (2014-09-04). "Dihydrolevoglucosenone (Cyrene) as a bio-based alternative for dipolar aprotic solvents". Chem. Commun. 50 (68): 9650–9652. doi:10.1039/c4cc04133j. PMID 25007289.
- "(1S,5R)-6,8-dioxabicyclo[3.2.1]octan-4-one - Registration Dossier - ECHA". echa.europa.eu. European Chemicals Agency, Europa (web portal). Retrieved 2019-01-31.
- Clark, James H.; Hunt, Andrew J.; Raverty, Warwick; Duncan, Tony; Farmer, Thomas J.; McElroy, C. Rob; Moity, Laurianne; Constantinou, Andri; Bruyn, Mario De (2014-07-29). "Dihydrolevoglucosenone (Cyrene) as a bio-based alternative for dipolar aprotic solvents". Chemical Communications. 50 (68): 9650–9652. doi:10.1039/C4CC04133J. ISSN 1364-548X. PMID 25007289.
- Trahanovsky; et al. (December 5, 2002). "A Convenient Procedure for the Preparation of Levoglucosenone and Its Conversion to Novel Chiral Derivatives". Carbohydrate Synthons in Natural Products Chemistry. ACS Symposium Series. 841. pp. 21–31. doi:10.1021/bk-2003-0841.ch002. ISBN 978-0-8412-3740-7.
- Huber, George W.; Dumesic, James A.; Krishna, Siddarth H.; McClelland, Daniel J.; Schwartz, Thomas J.; Cao, Fei (2015-06-03). "Dehydration of cellulose to levoglucosenone using polar aprotic solvents". Energy & Environmental Science. 8 (6): 1808–1815. doi:10.1039/C5EE00353A. ISSN 1754-5706.
- Method for selectively preparing evoglucosenone (LGO) and other anhydrosugars from biomass in polar aprotic solvents, 2014-12-31, retrieved 2019-01-31
- Clark, J. H.; McQueen-Mason, S. J.; Raverty, W. D.; Farmer, T. J.; Simister, R.; Gomez, L. D.; Macquarrie, D. J.; Budarin, V. L.; Fan, J. (2016-08-02). "A new perspective in bio-refining: levoglucosenone and cleaner lignin from waste biorefinery hydrolysis lignin by selective conversion of residual saccharides". Energy & Environmental Science. 9 (8): 2571–2574. doi:10.1039/C6EE01352J. ISSN 1754-5706.
- Huber, George W.; Dumesic, James A.; Rashke, Quinn A.; McClelland, Daniel J.; Krishna, Siddarth H. (2017-03-06). "Hydrogenation of levoglucosenone to renewable chemicals". Green Chemistry. 19 (5): 1278–1285. doi:10.1039/C6GC03028A. ISSN 1463-9270. OSTI 1477850.
- Mazarío, Jaime; Parreño Romero, Míriam; Concepción, Patricia; Chávez-Sifontes, Marvin; Spanevello, Rolando A.; Comba, María B.; Suárez, Alejandra G.; Domine, Marcelo E. (2019-07-26). "Tuning zirconia-supported metal catalysts for selective one-step hydrogenation of levoglucosenone". Green Chemistry. 21 (17): 4769–4785. doi:10.1039/C9GC01857C. ISSN 1463-9270.
- Shuttleworth, P. S.; Clark, J. H.; Ellis, G. J.; Budarin, V. L.; Bruyn, M. De; Sherwood, J.; Salavagione, H. J. (2017-06-06). "Identification of high performance solvents for the sustainable processing of graphene" (PDF). Green Chemistry. 19 (11): 2550–2560. doi:10.1039/C7GC00112F. ISSN 1463-9270.
- "Circa Data Sheet" (PDF). Archived (PDF) from the original on 2018-01-09.
- Method of producing (S)-4-hydroxymethyl-γ-lactone, 1990-09-17, retrieved 2019-01-31
- Okabe, Masami; Sun, Ruen Chu; Tam, Steve Y. K.; Todaro, Louis J.; Coffen, David L. (2002-05-01). "Synthesis of the dideoxynucleosides "ddC" and "CNT" from glutamic acid, ribonolactone, and pyrimidine bases". The Journal of Organic Chemistry. 53 (20): 4780–4786. doi:10.1021/jo00255a021.
- Process for preparing 1,6-hexanediol, 2013-04-25, retrieved 2019-01-31
- Huber, George W.; Dumesic, James A.; Hermans, Ive; Maravelias, Christos T.; Banholzer, Williams F.; Walker, Theodore; Burt, Samuel P.; Brentzel, Zachary J.; Alonso, David M. (2017-09-20). "New catalytic strategies for α,ω-diols production from lignocellulosic biomass". Faraday Discussions. 202: 247–267. Bibcode:2017FaDi..202..247H. doi:10.1039/C7FD00036G. ISSN 1364-5498. PMID 28678237. S2CID 39560658.
- Byrne, Fergal P.; Jin, Saimeng; Paggiola, Giulia; Petchey, Tabitha H. M.; Clark, James H.; Farmer, Thomas J.; Hunt, Andrew J.; Robert McElroy, C.; Sherwood, James (2016-05-23). "Tools and techniques for solvent selection: green solvent selection guides". Sustainable Chemical Processes. 4 (1): 7. doi:10.1186/s40508-016-0051-z. ISSN 2043-7129.
- Watson, Allan J. B.; Jamieson, Craig; Greatrex, Ben; Murray, Jane; Kennedy, Alan R.; Wilson, Kirsty L. (2016-09-08). "Scope and limitations of a DMF bio-alternative within Sonogashira cross-coupling and Cacchi-type annulation". Beilstein Journal of Organic Chemistry. 12 (1): 2005–2011. doi:10.3762/bjoc.12.187. ISSN 1860-5397. PMC 5082449. PMID 27829904.
- Watson, Allan J. B.; Jamieson, Craig; Murray, Jane; Wilson, Kirsty L. (2017-12-11). "Cyrene as a Bio-Based Solvent for the Suzuki–Miyaura Cross-Coupling". Synlett. 29 (5): 650–654. doi:10.1055/s-0036-1589143. ISSN 0936-5214.
- Camp, Jason E.; Bousfield, Thomas W.; Mapesa, Kopano; Mistry, Liam (2017-05-08). "Synthesis of ureas in the bio-alternative solvent Cyrene" (PDF). Green Chemistry. 19 (9): 2123–2128. doi:10.1039/C7GC00908A. ISSN 1463-9270.
- Gyekye, Liz. "Circa gets thumbs up from EU to sell its bio-based solvent in the bloc". www.biobasedworldnews.com. Archived from the original on 2019-01-05. Retrieved 2019-01-31.