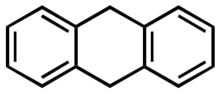
9,10-Dihydroanthracene
9,10-Dihydroanthracene is an organic compound that is derived from the polycyclic aromatic hydrocarbon anthracene. Several isomers of dihydroanthracene are known, but the 9,10 derivative is most common. It is a colourless solid that is used as a carrier of H2 as a hydrogen-donor.[1]
 | |
| Names | |
|---|---|
| Preferred IUPAC name
9,10-Dihydroanthracene | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.009.398 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C14H12 | |
| Molar mass | 180.250 g·mol−1 |
| Appearance | white solid |
| Density | 0.88 g mL−1 |
| Melting point | 108 to 109 °C (226 to 228 °F; 381 to 382 K) |
| Boiling point | 312 °C (594 °F; 585 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation
Because the aromaticity is not compromised for the flanking rings, anthracene is susceptible to hydrogenation at the 9- and 10- positions. It is produced in the laboratory by dissolving metal reduction using sodium/ethanol,[2] an application of the Bouveault–Blanc reduction developed by Louis Bouveault and Gustave Louis Blanc in 1903.[3][4][5] The reduction can be effected by magnesium as well. Finally, it can also be prepared by the coupling of benzyl chloride using aluminium chloride as a catalyst.
The bond dissociation energy for the 9- and 10- carbon–hydrogen bonds are estimated at 78 kcal mol−1. Thus these bonds are about 20% weaker than typical C–H bonds.
References
- Collin, Gerd; Höke, Hartmut; Talbiersky, Jörg (2006). "Anthracene". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a02_343.pub2.
- Bass, K. C. (1962). "9,10-Dihydroanthracene". Organic Syntheses. 42: 48. doi:10.15227/orgsyn.042.0048.; Collective Volume, 5, p. 398
- Bouveault, Louis; Blanc, Gustave Louis (1903). "Préparation des alcools primaires au moyen des acides correspondants" [Preparation of primary alcohols by means of the corresponding acids]. Compt. Rend. (in French). 136: 1676–1678.
- Bouveault, Louis; Blanc, Gustave Louis (1903). "Préparation des alcools primaires au moyen des acides correspondants" [Preparation of primary alcohols by means of the corresponding acids]. Compt. Rend. (in French). 137: 60–62.
- Bouveault, Louis; Blanc, Gustave Louis (1904). "Transformation des acides monobasiques saturés dans les alcools primaires correspondants" [Transforming saturated monobasic acids into the corresponding primary alcohols]. Bull. Soc. Chim. Fr. (in French). 31: 666–672.