Dieckmann condensation
The Dieckmann condensation is the intramolecular chemical reaction of diesters with base to give β-keto esters.[1] It is named after the German chemist Walter Dieckmann (1869–1925).[2][3] The equivalent intermolecular reaction is the Claisen condensation.
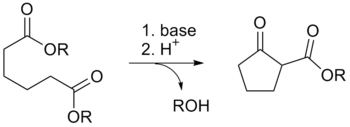 The Dieckmann condensation
The Dieckmann condensation
| Dieckmann condensation | |
|---|---|
| Named after | Walter Dieckmann |
| Reaction type | Ring forming reaction |
| Identifiers | |
| Organic Chemistry Portal | dieckmann-condensation |
| RSC ontology ID | RXNO:0000065 |
Reaction mechanism
Deprotonation of an ester at the α-position generates an enolate ion which then undergoes a 5-exo-trig nucleophilic attack to give a cyclic enol. Protonation with a Brønsted-Lowry acid (H3O+ for example) re-forms the β-keto ester.[4]
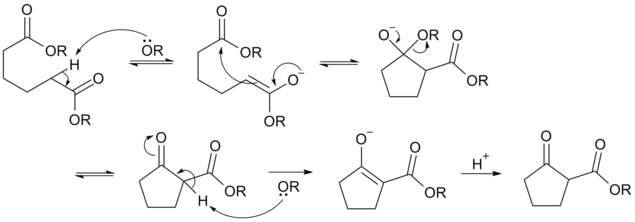 Dieckmann Condensation reaction mechanism for the example given.
Dieckmann Condensation reaction mechanism for the example given.
Due to the steric stability of five- and six-membered rings, these structures will preferentially be formed. 1,6 diesters will form five-membered cyclic β-keto esters, while 1,7 diesters will form six-membered β-keto esters.[5]
 |
| Animation of the reaction mechanism |
Further reading
- Dieckmann, W. Ber. 1894, 27, 102 & 965
- Dieckmann, W. Chemische Berichte|Ber. 1900, 33, 595 & 2670
- Dieckmann, W. Ann. 1901, 317, 51 & 93
gollark: What *is* a tensor, anyway?
gollark: Oh bees.
gollark: Oh no.
gollark: What *is* the interdimensional stability constant these days?
gollark: Ah yes, the spaghnum constant?
See also
- Claisen condensation
- Gabriel-Colman rearrangement
- Thorpe–Ziegler reaction
References
- Davis, B. R.; Garrett, P. J. Compr. Org. Synth. 1991, 2, 806-829. (Review)
- Kwart, Harold; King, Kenneth (1969). "Rearrangement and cyclization reactions of carboxylic acids and esters". In S. Patai (ed.). PATAI'S Chemistry of Functional Groups: Carboxylic Acids and Esters (1969). pp. 341–373. doi:10.1002/9780470771099.ch8. ISBN 9780470771099.
- Schaefer, J. P.; Bloomfield, J. J. (1967). "The Dieckmann Condensation (Including the Thorpe-Ziegler Condensation)". Organic Reactions. 15: 1–203. doi:10.1002/0471264180.or015.01.
- Janice Gorzynski Smith (2007). Organic Chemistry (2nd ed.). pp. 932–933. ISBN 978-0073327495.
- "Dieckmann Condensation". Organic Chemistry Portal.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.