Bis(triphenylphosphine)palladium chloride
Bis(triphenylphosphine)palladium chloride is a coordination compound of palladium containing two triphenylphosphine and two chloride ligands. It is a yellow solid that is soluble in some organic solvents. It is used for palladium-catalyzed coupling reactions, e.g. the Sonogashira–Hagihara reaction. The complex is square planar. Many analogous complexes are known with different phosphine ligands.
palladium(II)-2D.png) | |
palladium(II)-from-xtal-3D-balls.png) | |
palladium(II).jpg) | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.034.299 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C36H30Cl2P2Pd | |
| Molar mass | 701.90 g·mol−1 |
| Appearance | yellow powder |
| Melting point | 260°C (decomposed around 300°C) |
| Insoluble in water, acetone, ether, Carbon tetrachloride and n-heptane // Soluble in CHCl3 and CH2Cl2, Chloroform (Slightly), Methanol (Slightly, Heated) | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | 181.7°C |
| Related compounds | |
Related compounds |
Bis(triphenylphosphine)platinum chloride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation and reactions
This compound may be prepared by treating palladium(II) chloride with triphenylphosphine:[1][2]
- PdCl2 + 2 PPh3 → PdCl2(PPh3)2
Upon reduction with hydrazine in the presence of excess triphenylphosphine, the complex is a precursor to tetrakis(triphenylphosphine)palladium, Pd(PPh3)4:[3]
- 2 PdCl2(PPh3)2 + 4 PPh3 + 5 N2H4 → 2 Pd(PPh3)4 + N2 + 4 N2H5+Cl−
Structure
Several crystal structures containing PdCl2(PPh3)2 have been reported. In all of the structures, PdCl2(PPh3)2 adopts a square planar coordination geometry and the trans isomeric form.[4][5][6][7]
Applications
The complex is used as a pre-catalyst for a variety of coupling reactions.[8]
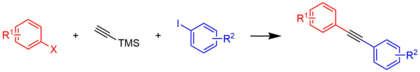
The Suzuki reaction was once limited by high levels of catalyst and the limited availability of boronic acids. Replacements for halides were also found, increasing the number of coupling partners for the halide or pseudohalide as well. Using bis(triphenylphosphine)palladium chloride as the catalyst, triflates and boronic acids have been coupled on an 80 kilogram scale in good yield.[9] The same catalyst is effective for the Sonogashira coupling.[10]
See also
- Bis(triphenylphosphine)platinum(II) chloride
- Bis(triphenylphosphine)nickel(II) chloride
References
- Norio Miyaura and Akira Suzuki (1990). "Palladium-Catalyzed Reaction of 1-Alkenylboronates with Vinylic Halides: (1Z,3E)-1-Phenyl-1,3-octadiene". Organic Syntheses. 68: 130. doi:10.15227/orgsyn.068.0130.CS1 maint: uses authors parameter (link)
- Hiroshi Itatani, J.C.Bailar (1967). "Homogeneous Catalysis in the Reactions of olefinic Substances. V.Hydrogenation of Soybean Oil Methyl Ester with Triphenylphosphine and Triphenylarsine Palladium Catalysts". Journal of the American Oil Chemists' Society. 44: 147. doi:10.1007/BF02558176.CS1 maint: uses authors parameter (link)
- D. R. Coulson (1972). Tetrakis(triphenylphosphine)palladium(0). Inorg. Synth. Inorganic Syntheses. 13. pp. 121–124. doi:10.1002/9780470132449.ch23. ISBN 9780470132449.
- G. Ferguson, R. McCrindle, A. J. McAlees and M. Parvez (1982). "trans-Dichlorobis(triphenylphosphine)palladium(II)". Acta Crystallogr. B38 (10): 2679–2681. doi:10.1107/S0567740882009583.CS1 maint: uses authors parameter (link)
- G. Steyl (2006). "trans-Dichlorobis(triphenylphosphine)palladium(II) dichloromethane solvate". Acta Cryst. E. 62: m1324–m1325. doi:10.1107/S1600536806017521.
- J. Pons, J. García-Antón, X. Solans, M. Font-Bardia, J. Ros (2008). "trans-Dichloridobis(triphenylphosphine)palladium(II)". Acta Cryst. E. 64: m621. doi:10.1107/S1600536808008337.CS1 maint: uses authors parameter (link)
- A. Naghipour, A. Ghorbani-Choghamarani, H. Babaee, M. Hashemi, B. Notash (2017). "Crystal structure of a novel polymorph of trans-dichlorobis (triphenylphosphine) palladium (II) and its application as a novel, efficient and retrievable catalyst for the amination of aryl halides and stille cross-coupling reactions". J. Organomet. Chem. 841: 31–38. doi:10.1016/j.jorganchem.2016.10.002.CS1 maint: uses authors parameter (link)
- René Severin, Jessica Reimer, Sven Doye (2010). "One-Pot Procedure for the Synthesis of Unsymmetrical Diarylalkynes". J. Org. Chem. 75 (10): 3518–352. doi:10.1021/jo100460v. PMID 20420397.CS1 maint: uses authors parameter (link)
- Jacks, T. E.; Belmont, Daniel T.; Briggs, Christopher A.; Horne, Nicole M.; Kanter, Gerald D.; Karrick, Greg L.; Krikke, James J.; McCabe, Richard J.; Mustakis; Nanninga, Thomas N. (2004). "Development of a Scalable Process for CI-1034, an Endothelin Antagonist". Organic Process Research & Development. 8 (2): 201–212. doi:10.1021/op034104g.CS1 maint: uses authors parameter (link)
- Chinchilla, R.; Nájera, C. (2007). "The Sonogashira Reaction: A Booming Methodology in Synthetic Organic Chemistry". Chem. Rev. 107 (3): 874–922. doi:10.1021/cr050992x. PMID 17305399.CS1 maint: uses authors parameter (link)
