Dicarbollide
In organometallic chemistry, a dicarbollide is an anion of the formula [C2B9H11]2-. Various isomers exist, but most common is 1,2-dicarbollide derived from ortho-carborane.[2] These dianions function as ligands, related to the cyclopentadienyl anion. Substituted dicarbollides are also known such as [C2B9H10(pyridine)]− (pyridine bonded to B) and [C2R2B9H9]2- (R groups bonded to carbon).
Synthesis of dicarbollides
Dicarbollides are obtained by base-degradation of 12-vertex dicarboranes. This degradation of the ortho derivative has been most heavily studied. The conversion is conducted in two-steps, first "deboronation" and second deprotonation:[3]
_redox.png)
- C2B10H12 + NaOEt + 2 EtOH → Na+C2B9H12− + H2 + B(OEt)3
- Na+C2B9H12− + NaH → Na2C2B8H11 + H2
The dianion derived from ortho-carborane, [C2B9H11]2- is a nido cluster. The nomenclature rules call the high coordination number vertex as 1. Thus the nido cluster with two adjacent carbon centers on the rim is the 7,8-isomer.
Coordination compounds
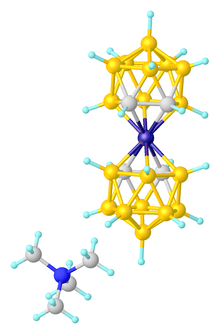
A variety of complexes are known with one or two dicarbollide ligands. An example of a 1:1 complex is [Mn(CO)3(η5-7,8-C2B9H11)]−.[4]
Most heavily studied are complexes with two dicarbollide ligands, especially sandwich complexes. Thus, these are prepared by salt metathesis reactions, as illustrated by the synthesis of the ferrocene analogue:
- 2 Na2C2B8H11 + FeCl2 → Na2[Fe(C2B8H11)2] + 2 NaCl
These bisdicarbollide dianions are often readily oxidized. Fe(III), Co(III), Ni(III), and Ni(IV) derivatives are known. In some cases, the oxidation induces rearrangement of the C2B9 cage to give complexes where the carbon centers are nonadjacent.[2]
References
- Kang, H. C.; Lee, S. S.; Knobler, C. B.; Hawthorne, M. F. (1991). "Syntheses of Charge-Compensated Dicarbollide Ligand Precursors and Their Use in the Preparation of Novel Metallacarboranes". Inorganic Chemistry. 30: 2024–2031. doi:10.1021/ic00009a015.CS1 maint: uses authors parameter (link)
- Sivaev, I. B.; Bregadze, V. I. (2000). "Chemistry of Nickel and Iron Bis(dicarbollides). A Review". Journal of Organometallic Chemistry. 614-615: 27–36. doi:10.1016/S0022-328X(00)00610-0.CS1 maint: uses authors parameter (link)
- Plešek, J.; Heřmánek, S.; Štíbr, B. (1983). "Potassium dodecahydro-7,8-dicarba-nido-undecaborate(1-), k[7,8-C2B9H12], intermediates, stock solution, and anhydrous salt". Inorganic Syntheses. 22: 231–234. doi:10.1002/9780470132531.ch53.
- Mitsuhiro Hata, Jason A. Kautz, Xiu Lian Lu, Thomas D. McGrath, F. Gordon A. Stone (2004). "Revisiting [Mn(CO)3(η5-nido-7,8-C2B9H11)]−, the Dicarbollide Analogue of [(η5-C5H5)Mn(CO)3]: Reactivity Studies Leading to Boron Atom Functionalization". Organometallics. 23: 3590–3602. doi:10.1021/om049822l.CS1 maint: uses authors parameter (link)