Diazolidinyl urea
Diazolidinyl urea is an antimicrobial preservative used in cosmetics. It is chemically related to imidazolidinyl urea which is used in the same way. Diazolidinyl urea acts as a formaldehyde releaser.
 newly determined structure | |
 "traditional" structure | |
| Names | |
|---|---|
| IUPAC names
1-[3,4-bis(hydroxymethyl)-2,5-dioxoimidazolidin-4-yl]-1,3-bis(hydroxymethyl)urea (new) 1-[1,3-bis(hydroxymethyl)-2,5-dioxoimidazolidin-4-yl]-1,3-bis(hydroxymethyl)urea (old) | |
| Other names
Diazolidinylurea Germall II | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.071.732 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H14N4O7 | |
| Molar mass | 278.22 g/mol |
Hazards | |
| GHS Signal word | Warning |
GHS hazard statements |
H317 |
| P261, P272, P280, P302+352, P333+313, P321, P363, P501 | |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Related compounds |
Imidazolidinyl urea |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
It is used in many cosmetics, skin care products, shampoos and conditioners, as well as a wide range of products including bubble baths, baby wipes and household detergents. Diazolidinyl urea is found in the commercially available preservative Germaben.
Commercial diazolidinyl urea is a mixture of different formaldehyde addition products including polymers.[2]
Chemistry
Synthesis
Diazolidinyl urea is produced by the chemical reaction of allantoin and formaldehyde in the presence of sodium hydroxide solution and heat. The reaction mixture is then neutralized with hydrochloric acid and evaporated:

Structure
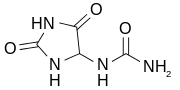
Diazolidinyl urea was poorly characterized until recently and the single Chemical Abstracts Service structure assigned to it is probably not the major one in the commercial material. Instead, new data indicate that one of the hydroxymethyl functional groups of the imidazolidine ring is attached to the carbon, rather than on the urea nitrogen atom:[2]
 |
 |
| Originally reported structure | Hoeck's revised structure |
Safety
Some people have a contact allergy to imidazolidinyl urea causing dermatitis.[3] Such people are often also allergic to diazolidinyl urea.
In addition to being an allergen, it is a formaldehyde releaser, meaning it releases the carcinogen formaldehyde slowly as it degrades
In 2005–06, it was the 14th-most-prevalent allergen in patch tests (3.7%).[4]
References
- HSNO Chemical Classification Information Database, New Zealand Environmental Risk Management Authority, retrieved 2009-09-06
- Lehmann, Søren Vig; Hoeck, Ulla; Breinholdt, Jens; Olsen, Carl Erik; Kreilgaard, Bo (2006). "Characterization and chemistry of imidazolidinyl urea and diazolidinyl urea". Cont. Dermat. 54 (1): 50–58. doi:10.1111/j.0105-1873.2006.00735.x. PMID 16426294.
- Review of toxicological data (NTP NIEHS)
- Zug KA, Warshaw EM, Fowler JF Jr, Maibach HI, Belsito DL, Pratt MD, Sasseville D, Storrs FJ, Taylor JS, Mathias CG, Deleo VA, Rietschel RL, Marks J. Patch-test results of the North American Contact Dermatitis Group 2005–2006. Dermatitis. 2009 May–Jun;20(3):149-60.
