Diamidophosphate
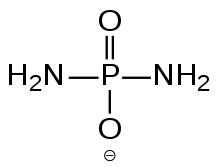
Diamidophosphate (DAP) is the simplest phosphorodiamidate ion, with formula PO2(NH2)2−. It is a phosphorylating ion and was first used for phosphorylation of sugars in aqueous medium.[1] Diamidophosphate can form salts such as sodium diamidophosphate, or an acid phosphorodiamidic acid. Phosphorodiamidic acid can crystallize as a trihydrate.[2] It is hypothesized as a plausible primordial reagent in the emergence of the first peptides, cell membrane lipids and nucleotides, the precursors of all life on Earth.[3] In a November 6, 2017 press release from the Scripps Research Institute, DAP was described as "a compound that may have been a crucial factor in the origins of life on Earth".[4] Other nitrogenous derivatives of phosphorus derivatives have also been proposed in this context in a review article.[5]
 | |
| Names | |
|---|---|
| IUPAC name
diaminophosphinate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| H4N2O2P | |
| Molar mass | 95.018 g·mol−1 |
| Related compounds | |
Other anions |
Thiophosphordiamidic acid |
Other cations |
Phosphordiamidic acid |
Related |
phosphorotriamide phosphoramidic acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Production
Diamidophosphate compounds can be made from phenyl diamidophosphate (phenylphosphorodiamidate) reacting with sodium hydroxide in a water solution. This solution can crystallise sodium diamidophosphate. Phosphorodiamidic acid trihydrate can be precipitated from the solution by adding it to an excess of ethanol.[2]
Reactions
The sodium salt crystallises as a hexahydrate. It can be dehydrated by heating at 70°C for a week.[6]
When anhydrous sodium diamidophosphate is heated it polmerises. At 160°C Na2P2O4(NH)(NH2)2, Na3P3O6(NH)2(NH2)2, Na4P4O8(NH)3(NH2)2, Na5P5O10(NH)4(NH2)2 and Na6P6O12(NH)5(NH2)2 are produced. These substances contain a P-N-P backbone. These can be separated by paper chromatography.[6] At 200°C heat the hexa-phosphate is the most common and at 250°C the typical chain length is 18.[6] If hydrated salts are strongly heated they lose ammonia to form oligophosphates and polyphosphates.[6] This compares with the heating of sodium amidophosphate, which yields sodium imidodiphosphate Na4P2O6NH and sodium nitridotriphosphate Na6P3O9N is produced also.[6]
Diamidophosphate binds to nickel ions, and inhibits urease enzymes by blocking up the active site, binding to two nickel atoms. Diamidophosphate mimics the urea hydrolysis intermediate.[7]
A silver AgPO2(NH2)2 and a potassium diamidophosphate salt KPO2(NH2)2 are also known. The silver salt can react using double decomposition with bromides forming other salts.[6]
Diamidophosphate can also be tribasic, and the amine groups may also lose hydrogen to form more metallic salts. With silver further reactions can yield explosive salts: tetrasilver orthodiamidophosphate (AgO)3P(NH2)NHAg, and pentasilver orthodiamidophosphate (AgO)3P(NHAg)2.[8]
Numerous organic derivatives are known. Organic groups can substitute on the oxygen for the OH group, or replace a hydrogen on the amine group.[9]
References
- Krishnamurthy, Ramanarayanan; Guntha, Sreenivasulu; Eschenmoser, Albert (4 July 2000). "Regioselective α-Phosphorylation of Aldoses in Aqueous Solution". Angewandte Chemie International Edition. 39 (13): 2281–2285. doi:10.1002/1521-3773(20000703)39:13<2281::AID-ANIE2281>3.0.CO;2-2. ISSN 1521-3773. PMID 10941064.
- Coggins, Adam J.; Powner, Matthew W. (10 October 2016). "Prebiotic synthesis of phosphoenol pyruvate by α-phosphorylation-controlled triose glycolysis Supplementary Information Compound 8" (PDF). Nature Chemistry. 9 (4): 310–317. Bibcode:2017NatCh...9..310C. doi:10.1038/nchem.2624. ISSN 1755-4349. PMID 28338685.
- "Phosphorylation, Oligomerization and Self-assembly in Water Under Potential Prebiotic Conditions", Gibard et al, Nature Chemistry (2017) doi:10.1038/nchem.2878, published online 06 November 2017
- "Scientists Find Potential "Missing Link" in Chemistry That Led to Life on Earth". Scripps Research Institute. November 6, 2017. Retrieved 7 November 2017.
- Karki, Megha; Gibard, Clémentine; Bhowmik, Subhendu; Krishnamurthy, Ramanarayanan (2017-07-29). "Nitrogenous Derivatives of Phosphorus and the Origins of Life: Plausible Prebiotic Phosphorylating Agents in Water". Life. 7 (3): 32. doi:10.3390/life7030032. PMC 5617957. PMID 28758921.
- Klement, R.; Biberacher, G. (May 1956). "Das thermische Verhalten von Natriumdiamidophosphat, Darstellung von kondensierten Imidophosphaten". Zeitschrift für Anorganische und Allgemeine Chemie. 285 (1–2): 74–85. doi:10.1002/zaac.19562850109.
- Zamble, Deborah; Rowińska-Żyrek, Magdalena; Kozlowski, Henryk (2017). The Biological Chemistry of Nickel. Royal Society of Chemistry. pp. 73–74, 83. ISBN 9781788010580.
- Bretherick, L. (2016). Bretherick's Handbook of Reactive Chemical Hazards. Elsevier. p. 19. ISBN 9781483162508.
- Kiss, S.; Simihaian, M. (2013). Improving Efficiency of Urea Fertilizers by Inhibition of Soil Urease Activity. Springer Science & Business Media. pp. 105–108. ISBN 9789401718431.
See also
Other reading
- H. N. Stokes (1894). "On Diamidoorthophosphoric and Diamidotrihydroxyphosphoric Acids". American Chemical Journal. 16 (2): 123.