1D-chiro-Inositol
1D-chiro-Inositol[2] (formerly D-chiro-inositol, commonly abbreviated DCI) is a member of a family of related substances often referred to collectively as "inositol", although that term encompasses several isomers of questionable biological relevance, including 1L-chiro-inositol. myo-Inositol is converted into DCI by an insulin dependent NAD/NADH epimerase enzyme.[3][4][5][6][7] It is known to be an important secondary messenger in insulin signal transduction. DCI accelerates the dephosphorylation of glycogen synthase and pyruvate dehydrogenase, rate limiting enzymes of non-oxidative and oxidative glucose disposal. DCI may act to bypass defective normal epimerization of myo-inositol to DCI associated with insulin resistance and at least partially restore insulin sensitivity and glucose disposal.[8]
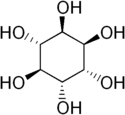 | |
| Names | |
|---|---|
| IUPAC name
1D-chiro-Inositol[2] | |
| Systematic IUPAC name
(1R,2R,3S,4S,5S,6S)-cyclohexane-1,2,3,4,5,6-hexol | |
| Other names | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.010.359 |
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H12O6 | |
| Molar mass | 180.156 g·mol−1 |
| Melting point | 230 °C (446 °F; 503 K) |
Chiral rotation ([α]D) |
[α]23/D +55°, c = 1.2 in H2O |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
References
- Merck Index, 11th Edition, 4883
- IUPAC Chemical Nomenclature and Structure Representation Division (2013). "P-104.2.1". In Favre, Henri A.; Powell, Warren H. (eds.). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. IUPAC–RSC. ISBN 978-0-85404-182-4.
- Sortino, Maria A.; Salomone, Salvatore; Carruba, Michele O.; Drago, Filippo (2017-06-08). "Polycystic Ovary Syndrome: Insights into the Therapeutic Approach with Inositols". Frontiers in Pharmacology. 8. doi:10.3389/fphar.2017.00341. ISSN 1663-9812. PMC 5463048. PMID 28642705.
- Kalra, Bharti; Kalra, Sanjay; Sharma, J. B. (2016). "The inositols and polycystic ovary syndrome". Indian Journal of Endocrinology and Metabolism. 20 (5): 720–724. doi:10.4103/2230-8210.189231. ISSN 2230-8210. PMC 5040057. PMID 27730087.
- Nestler, John E.; Unfer, Vittorio (July 2015). "Reflections on inositol(s) for PCOS therapy: steps toward success". Gynecological Endocrinology. 31 (7): 501–505. doi:10.3109/09513590.2015.1054802. ISSN 1473-0766. PMID 26177098.
- Bizzarri, M.; Carlomagno, G. (July 2014). "Inositol: history of an effective therapy for Polycystic Ovary Syndrome". European Review for Medical and Pharmacological Sciences. 18 (13): 1896–1903. ISSN 2284-0729. PMID 25010620.
- Heimark, Douglas; McAllister, Jan; Larner, Joseph (2014). "Decreased myo-inositol to chiro-inositol (M/C) ratios and increased M/C epimerase activity in PCOS theca cells demonstrate increased insulin sensitivity compared to controls". Endocrine Journal. 61 (2): 111–117. ISSN 1348-4540. PMID 24189751.
- Larner, Joseph (2002). "D-chiro-inositol—its functional role in insulin action and its deficit in insulin resistance". International Journal of Experimental Diabetes Research. 3 (1): 47–60. doi:10.1080/15604280212528. ISSN 1560-4284. PMC 2478565. PMID 11900279.