Cyclopropane fatty acid
Cyclopropane fatty acids (CPA) are a subgroup of fatty acids that contain a cyclopropane group.[1] Although they are usually rare, the seed oil from lychee contains nearly 40% CPAs in the form of triglycerides.[2]
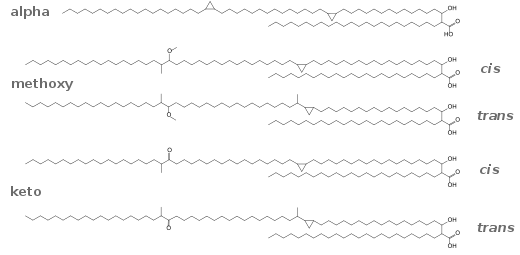
Biosynthesis
CPAs are derived from unsaturated fatty acids by cyclopropanation. The methylene donor is a methyl group on S-adenosylmethionine (SAM). The conversion is catalyzed by cyclopropane-fatty-acyl-phospholipid synthase.[3] The mechanism is proposed to involve transfer of a CH3+ group from SAM to the alkene, followed by deprotonation of the newly attached methyl group and ring closure.[4]
Cyclopropene fatty acids
Cyclopropene fatty acids are even rarer than CPAs. The best-known examples are malvalic acid and sterculic acid. Sterculic acid as its triglyceride is present in sterculia oils and at low levels in kapok seed oil (~12%), cottonseed oil (~1%), and in the seeds of the tree Sterculia foetida (~65-78%). These acids are highly reactive but the cyclopropene ring is destroyed during refining and hydrogenation of the oils. They have attracted interest because they reduce levels of the enzyme stearoyl-CoA 9-desaturase (SCD), which catalyzes the biodesaturation of stearic acid to oleic acid.[5]
At least one review indicates that CPFA are carcinogenic, co-carcinogenic, and have medical and other effects on animals;[6] according to this review, "CPFA in food is dangerous to human health".
References
- "Archived copy". Archived from the original on 2014-12-17. Retrieved 2015-02-02.CS1 maint: archived copy as title (link)
- Gaydou, E. M.; Ralaimanarivo, A.; Bianchini, J. P. "Cyclopropanoic Fatty-Acids of Litchi (Litchi-Chinensis) Seed Oil - a Reinvestigation" J. Agr. Food Chem. 1993, vol. 41, pp. 886-890. doi:10.1021/jf00030a009
- Grogan DW, Cronan JE Jr: Cyclopropane ring formation in membrane lipids of bacteria" Microbiol Mol Biol Rev 1997, vol. 61, pp. 429-441. http://mmbr.asm.org/content/61/4/429.full.pdf+html
- Ludger A. Wessjohann and Wolfgang Brandt, Thies Thiemann "Biosynthesis and Metabolism of Cyclopropane Rings in Natural Compounds" Chem. Rev., 2003, volume 103, pp 1625–1648. doi:10.1021/cr0100188
- Abraham, Samuel (1975). International encyclopedia of pharmacology and therapeutics: Pharmacology of lipid transport and atherosclerotic processes, Volume 1. Pergamon Press. p. 108. ISBN 9780080177625.
- L. O. Hanus, P. Goldshlag, V. M. Dembitsky (2008). IDENTIFICATION OF CYCLOPROPYL FATTY ACIDS IN WALNUT (JUGLANS REGIA L.) OIL. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2008, 152(1):41–45.
External links
- Bao, X.; Katz, S.; Pollard, M.; Ohlrogge, J. (2002). "Carbocyclic fatty acids in plants: Biochemical and molecular genetic characterization of cyclopropane fatty acid synthesis of Sterculia foetida" (PDF). Proceedings of the National Academy of Sciences. 99 (10): 7172–7177. Bibcode:2002PNAS...99.7172B. doi:10.1073/pnas.092152999. PMC 124547. PMID 11997456.