Cyclopentadienone
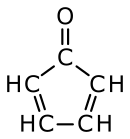
Cyclopentadienone is an organic compound with molecular formula C5H4O. The parent cyclopentadienone is rarely encountered, because it rapidly dimerizes.[1] Many substituted derivatives are known, notably tetraphenylcyclopentadienone. Such compounds are used as ligands in organometallic chemistry.[2]
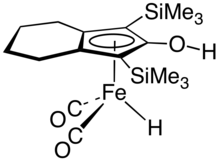
The Knölker complex, derived from a substituted cyclopentadienone, is a catalyst for transfer hydrogenation.[3]
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H4O | |
| Molar mass | 80.086 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation
Cyclopentadienone can be generated by the photolysis or pyrolysis of various substances (e.g. 1,2-benzoquinone[4]), and then isolated in an argon matrix at 10 K. It dimerizes readily upon thawing the matrix (38 K).[5][6]
gollark: No.
gollark: Do I now.
gollark: They *have firmware for* a specific task. They are general purpose computers.
gollark: Well, they can all independently execute code.
gollark: - actual CPU- power management microcontroller on CPU- Intel Management Engine- Intel GuC on CPU (graphics microcontroller)- Intel *H*uC (HEVC microcontroller)- WiFi card microcontroller- Ethernet chip probably has a processor in it- dedicated GPU onboard microcontroller- display panel probably has a processor too, definitely at least an EEPROM- laptop embedded controller for general purpose things- camera microcontroller for debayering and USB- keyboard USB controller
See also
References
- Michael A. Ogliaruso, Michael G. Romanelli, Ernest I. Becker "Chemistry of Cyclopentadienones" Chem. Rev., 1965, vol. 65, pp 261–367. doi:10.1021/cr60235a001
- Quintard, A.; Rodriguez, J., "Iron Cyclopentadienone Complexes: Discovery, Properties, and Catalytic Reactivity", Angew. Chem. Int. Ed. 2014, vol. 53, 4044-4055. doi:10.1002/anie.201310788
- Casey, Charles P.; Guan, Hairong (2007). "An Efficient and Chemoselective Iron Catalyst for the Hydrogenation of Ketones". Journal of the American Chemical Society. 129 (18): 5816–5817. doi:10.1021/ja071159f. PMID 17439131.
- Brown, Roger F. C. (2012). Pyrolytic Methods in Organic Chemistry: Application of Flow and Flash Vacuum Techniques. Elsevier. p. 173. ISBN 978-0-323-15417-8.
- Maier, Günther; Franz, Lothar Hermann; Hartan, Hans-Georg; Lanz, Klaus; Reisenauer, Hans Peter (1985). "Kleine Ringe, 54. Cyclopentadienon". Chem. Ber. (in German). 118 (8): 3196–3204. doi:10.1002/cber.19851180819.
- Horspool, William M.; Lenci, Francesco. CRC Handbook of Organic Photochemistry and Photobiology, Volumes 1 & 2 (2nd ed.). p. 12-8. ISBN 978-0-203-49590-2.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.