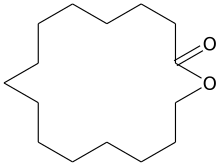
Cyclopentadecanolide
Cyclopentadecanolide is a natural macrolide lactone and a synthetic musk.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Oxacyclohexadecan-2-one | |
| Other names
Angelica lactone; Muskalactone; Muskolactone; Exaltolide; Pentalide; Pentadecanolide; Pentadecalactone; 15-Hydroxypentadecanoic acid, lactone; 15-Hydroxypentadecanoic acid-epsilon-lactone; Pentadecanoic acid, 15-hydroxy-, E-lactone; ω-Pentadecalactone; omega-Pentadecalactone; ω-Lactone; 2-Pentadecalone; Pentadecan-15-olide; 1,15-Pentadecanolide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.003.050 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C15H28O2 | |
| Molar mass | 240.387 g·mol−1 |
| Appearance | Colorless crystals |
| Odor | Musklike |
| Density | 0.940 |
| Melting point | 34 °C (93 °F; 307 K)[1] |
| Boiling point | 98 °C (208 °F; 371 K)[2] at 0.02 Torr |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Natural occurrence
Cyclopentadecanolide occurs in small quantities in angelica root essential oil and is responsible for its musklike odor.[3]
Production
Cyclopentadecanolide is produced synthetically by ring expansion of cyclotetradecanone. Another synthesis route is the depolymerization of polyesters of 15-hydroxypentadecanoic acid.[3]
Uses
Cyclopentadecanolide is used as a musklike perfume fixative in fine fragrances and as a flavoring agent.[4] It is a substitute for the extremely expensive animal musk.[3]
gollark: These CPU vulnerabilities really stem from the fact that they're trying to emulate decades-old systems well enough to run C-style programs (i.e. monotasking, for loops and not map etc everywhere, mutability *and* sharing memory...) properly while tacking on new features for speed etc.
gollark: I'm now selling synthetic dragon eggs at Wojbie's shop. 2KST/i.
gollark: Er...
gollark: Madness.
gollark: What are they?
References
- Morales-Serna, José Antonio; Sánchez, Ericka; Velázquez, Ricardo; Bernal, Jorge; García-Ríos, Eréndira; Gaviño, Rubén; Negrón-Silva, Guillermo; Cárdenas, Jorge (2010). "Highly efficient macrolactonization of ω-hydroxy acids using benzotriazole esters: synthesis of Sansalvamide A". Organic & Biomolecular Chemistry. 8 (21): 4940. doi:10.1039/c0ob00161a. ISSN 1477-0520.
- Bestmann, Hans Jürgen; Schobert, Rainer (1989). "Kumulierte Ylide XX.1Synthesen (E)-α,β-ungesättigter macrocyclischer Lactone durch intramolekulare Wittig-Olefinierung via Triphenylphosphoranylidenketen2". Synthesis (in German). 1989 (06): 419–423. doi:10.1055/s-1989-27271. ISSN 0039-7881. Archived from the original on 2018-06-05. Retrieved 2020-08-01.
- Karl-Georg Fahlbusch; et al. (2007), "Flavors and Fragrances", Ullmann's Encyclopedia of Industrial Chemistry (7th ed.), Wiley, p. 75
- George A. Burdock (2010), "ω-PENTADECALACTONE", Fenaroli's Handbook of Flavor Ingredients (6th ed.), Taylor & Francis, p. 1597
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.