Cuneane
Cuneane (C8H8, pentacyclo[3.3.0.02,4.03,7.06,8]octane) is a saturated hydrocarbon.[1] Its name is derived from the Latin cuneus, meaning a wedge.[2] Cuneane may be produced from cubane by metal-ion-catalyzed σ-bond rearrangement.[3][4] Similar reactions are known for homocubane (C9H10) and bishomocubane (C10H12).[5][6]
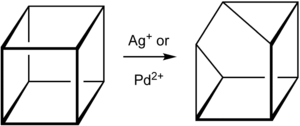 Scheme 1. Synthesis of cuneane from cubane
Scheme 1. Synthesis of cuneane from cubane
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H8 | |
| Molar mass | 104.152 g·mol−1 |
| Density | 1.578 g/ml |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Molecular geometry
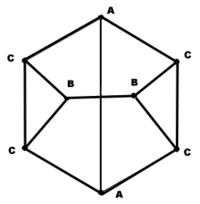
The carbon atoms in the cuneane molecule form a hexahedron with point group C2v. The cuneane molecule has three groups of equivalent carbon atoms (A, B, C), which have also been confirmed by NMR.[7] The molecular graph of the carbon skeleton of cuneane is a regular graph with non-equivalent groups of vertices, and so it is a very important test object for different algorithms of mathematical chemistry.[8][9]
Derivatives
Some cuneane derivatives have liquid crystal properties.[10]
References
- with 3D-structure (however molecular graph of cuneane is a planar graph)
- R. Criegee; R. Askani (1968). "Octamethylsemibullvalene". Angewandte Chemie International Edition in English. 7 (7): 537. doi:10.1002/anie.196805371.
- Michael B. Smith; Jerry March (2001). March’s Advanced Organic Chemistry (5th ed.). John Wiley & Sons, Inc. p. 1459. ISBN 0-471-58589-0.
- Philip E. Eaton; Luigi Cassar; Jack Halpern (1970). "Silver(I)- and palladium(II)-catalyzed isomerizations of cubane. Synthesis and characterization of cuneane". Journal of the American Chemical Society. 92 (21): 6366–6368. doi:10.1021/ja00724a061.
- Leo A. Paquette; John C. Stowell (1970). "Silver ion catalyzed rearrangements of strained sigma. bonds. Application to the homocubyl and 1,1'-bishomocubyl systems". Journal of the American Chemical Society. 92 (8): 2584–2586. doi:10.1021/ja00711a082.
- W. G. Dauben; M. G. Buzzolini; C. H. Schallhorn; D. L. Whalen; K. J. Palmer (1970). "Thermal and silver ion catalyzed isomerization of the 1,1′-bishomocubane system: preparation of a new C10H10isomer". Tetrahedron Letters. 11 (10): 787–790. doi:10.1016/S0040-4039(01)97830-X.
- H. Guenther; W. Herrig (1973). "Anwendungen der 13C-Resonanz-Spektroskopie, X. 13C,13C-Kopplungskonstanten in Methylencycloalkanen". Chemische Berichte. 106 (12): 3938–3950. doi:10.1002/cber.19731061217.
- M.I. Trofimov; E.A. Smolenskii (2000). "Electronegativity of atoms of ring-containing molecules—NMR spectroscopy data correlations: a description within the framework of topological index approach". Russian Chemical Bulletin. 49 (3): 402. doi:10.1007/BF02494766.
- M.I. Trofimov; E.A. Smolenskii (2005). "Application of the electronegativity indices of organic molecules to tasks of chemical informatics". Russian Chemical Bulletin. 54 (9): 2235. doi:10.1007/s11172-006-0105-6.
- Bényei, Gyula; Jalsovszky, István; Demus, Dietrich; Prasad, Krishna; Rao, Shankar; Vajda, Anikó; Jákli, Antal; Fodor‐Csorba, Katalin (2006). "First liquid crystalline cuneane‐caged derivatives: a structure-property relationship study". Liquid Crystals. 33 (6): 689–696. doi:10.1080/02678290600722940.