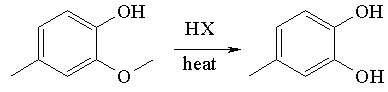Creosol
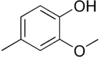
Creosol is a chemical compound with the molecular formula C8H10O2. It is one of the components of creosote. Compared with phenol, creosol is a less toxic disinfectant.
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
2-Methoxy-4-methylphenol | |||
| Other names
4-Methylguaiacol; Valspice | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
| ECHA InfoCard | 100.002.049 | ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C8H10O2 | |||
| Molar mass | 138.16 g/mol | ||
| Appearance | Colorless to yellowish aromatic liquid | ||
| Density | 1.0966 g/cm3 (20 °C) [1] | ||
| Melting point | 5.5 °C (41.9 °F; 278.6 K) | ||
| Boiling point | 221[1] °C (430 °F; 494 K) | ||
| Slightly soluble | |||
| Solubility in ethanol, ether, benzene | Miscible | ||
Refractive index (nD) |
1.5373 (20 °C) [1] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Sources
Sources of creosol include:
- Coal tar creosote
- Wood creosote
- Reduction product of vanillin using zinc powder in strong hydrochloric acid
- Found as glycosides in green vanilla beans[2]
- It is also found in tequila.[3]
Reactions
Creosol reacts with hydrogen halides to give a catechol.
gollark: They have files *internally*, obviously, but you can't do much with them.
gollark: Except phones and such basically do not files.
gollark: There are many arbitrary university rankings™.
gollark: Yes.
gollark: That never happens here.
See also
- Vanillin, a related phenol
References
- Baird, Zachariah Steven; Uusi-Kyyny, Petri; Pokki, Juha-Pekka; Pedegert, Emilie; Alopaeus, Ville (6 Nov 2019). "Vapor Pressures, Densities, and PC-SAFT Parameters for 11 Bio-compounds". International Journal of Thermophysics. 40 (11): 102. Bibcode:2019IJT....40..102B. doi:10.1007/s10765-019-2570-9.
- Dignum, Mark J.W.; Van Der Heijden, Rob; Kerler, Josef; Winkel, Chris; Verpoorte, Rob (2004). "Identification of glucosides in green beans of Vanilla planifolia Andrews and kinetics of vanilla β-glucosidase". Food Chemistry. 85 (2): 199–205. doi:10.1016/S0308-8146(03)00293-0.
- Characterization of volatile compounds from ethnic Agave alcoholic beverages by gas chromatography-mass spectrometry. León-Rodríguez, A. de, Escalante-Minakata, P., Jiménez-García, M. I., Ordoñez-Acevedo, L. G., Flores Flores, J. L. and Barba de la Rosa, A. P., Food Technology and Biotechnology, 2008, Volume 46, Number 4, pages 448-455 (abstract Archived 2014-03-13 at the Wayback Machine)
External links
![]()
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.