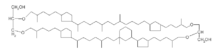
Crenarchaeol
Crenarchaeol is a glycerol dibiphantanyl glycerol tetraether (GDGT) biological membrane lipid. It has an distinctive cyclohexane moiety and has been proposed as a specific biomarker for pelagic ammonia-oxidizing archaea (AOA).[1] Structurally, the molecule consists of two long hydrocarbon chains that extend through the cell membrane and are bound on each to glycerol through ether linkage. Crenarchaeol can be preserved for hundreds of millions of years in the environment and is part of the TEX86 paleothermometer, a temperature proxy for sea surface temperatures that has been used to reconstruct paleoclimate through to the middle Jurassic (~160 Ma).[2]
 | |
| Names | |
|---|---|
| IUPAC name
[(9S,12S,16S,24S,28R,31R,35S,43S,46S,50S,58S,62R,65R)-12-(Hydroxymethyl)-9,16,24,28,31,35,43,50,58,62,65-undecamethyl-11,14,45,48-tetraoxahexacyclo[63.3.1.12,5.120,23.136,39.154,57]triheptacontan-46-yl]methanol | |
| Identifiers | |
3D model (JSmol) |
|
PubChem CID |
|
| |
| |
| Properties | |
| C82H154O6 | |
| Molar mass | 1236.128 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Discovery and distribution
Archaeal membranes are distinct from those of bacteria and eukaryotes because they contain isoprenoid GDGTs instead of diacyl lipids, which are found in the other domains. It has been proposed that GDGT membrane lipids are an adaptation to the high temperatures present in the environments that are home to extremophile archaea [1] and so researchers were met with surprise in 1997 when unknown archaeal GDGTs were detected in pelagic waters.[3] Unknown GDGTs were also found in marine sediments[4] and isolated from Cenarchaeum symbiosum,[5] a marine ammonia-oxidizing archaeon that lives in symbiosis with sponges.
Following the discovery of GDGTs outside of hydrothermal environments, crenarchaeol was first identified as the major GDGT component in surface sediments and extracts from C. symbiosum in 2002 by two-dimensional nuclear magnetic resonance (2D-NMR) spectroscopy.[6] It was named for the phylum Crenarchaeota, to which the ammonia-oxidizing pelagic archaea that produce crenarchaeol were thought to belong before it was proposed that the Marine Group I Crenarchaeota be considered a distinct phylum, Thaumarchaeota.[7]

Crenarchaeol is produced by AOA belonging to the phylum Thaumarchaeota (formerly classified as the Marine Group 1 Crenarchaeota). It has been confirmed to be produced by pure cultures of the pelagic mesothermic C. symbiosum[6] and Nitrosopumilus maritimus,[8] as well as the moderately thermophilic Nitrososphaera gargensis[1] and the hyperthrmophilic Candidatus Nitrosocaldus yellowstonii.[9] The discovery that crenarchaeol in Ca. N. yellowstonii and N. gargensis disproved the previous consensus that crenarchaeol was specific to mesothermic Thaumarchaeota and suggests that it is found more broadly within the phylum.
Controversy
One metagenomic study of the depth-distribution of archaeal clades in the South Pacific Gyre has suggested that crenarchaeol is not exclusive to the Thaumarchaeota, but is also produced by the Marine Group II Euryarchaeota.[10] However, no members of Marine Group II have thus far been cultivated[11] and conflicting environmental data continues to support the hypothesis that crenarchaeol is exclusive to the Thaumarchaeota.[12]
In biology
Chemistry and function
Like other GDGTs, crenarchaeol is a membrane lipid with distinct hydrophobic and hydrophilic regions. The long, nonpolar hydrocarbon chains are hydrophobic while the ether-linked glycerol head groups are polar and hydrophilic. In most organisms, the cell membrane consists of a lipid bilayer in which phospholipids arrange with their hydrophobic, nonpolar hydrocarbon tails facing inwards towards one another and their hydrophilic, polar head groups facing outwards to associate with the polar environments of the cytoplasm or cell exterior. This organization is promoted by the hydrophobic effect, which makes it energetically favorable for hydrophobic molecules to isolate themselves away from aqueous environments. Because GDGTs have two hydrophilic head groups, they form a lipid monolayer in the cell membrane instead of a bilayer, making GDGT-producing archaea exceptional among all clades of life.[13] Originally, it was believed that GDGT membrane lipids were an adaption to life at high temperatures and acidities. Because the two sides of a monolayer lipid are connected by covalent bonds rather than the weaker intermolecular forces that promote the cohesion of bilayers, they are more stable than typical bilayers.[13] This hypothesis is supported by the observation that some extremophile bacteria synthesize their own membrane-spanning, ether-bound GDGT analogues.[14] The cyclic moieties of GDGTs may also be an adaption to hyperthermal conditions,[6] and the number of rings in a GDGT's long hydrocarbon chains is temperature-dependent.[15] Crenarchaeol has two cyclopentyl moieties on one of its hydrocarbon chains and one cyclohexyl and two cyclopentyl moieties on the other.
However, the discovery that crenarchaeol and other GDGTs are produced by organisms living in mesothermal environments has thrown the hyperthermal-adaptation hypothesis into question.[13] It has been proposed that the distinctive cyclohexyl moiety of crenarchaeol is an adaption to pelagic life, as it produces a "kink" in one of crenarchaeol's hydrocarbon chains that prevents the membrane lipids from packing tightly, as would be favorable under high temperatures but unfavorable under temperate ones.[6]
Preservation and degradation in sediments
Crenarchaeol and other GDGTs can be preserved in the environment for hundreds of millions of years[2] under the right conditions. Most GDGTs degrade at between 240 and 300 °C and so are not found in rocks that have undergone heating to temperatures higher than 300 °C.[16] GDGTs undergo degradation when exposed to oxygen but the relative concentrations of sediment GDGTs tends to remain the same even during degradation, meaning that degradation does not interfere with proxies like TEX86[17] that are based on the ratios of different GDGTs.
Marine nitrogen cycle
Ammonia oxidation is important part of the nitrogen cycle, a biogeochemical cycle which cycles nitrogen through its various biological and mineral forms. AOA have been shown to dominate ammonia oxidation in the oceans[18][19] and thus crenarchaeol, which is generally thought to be produced exclusively by AOA (specifically Thaumarchaeota), has been proposed as a specific biomarker for AOA and ammonia oxidation. Crenarchaeol abundance has been found to track with seasonal blooms of AOA, suggesting that it may be appropriate to use crenarchaeol abundances as a proxy for AOA populations[20] and thus ammonia oxidation more broadly. However the discovery of Thaumarchaeota that are not obligate ammonia-oxidizers[21] complicates this conclusion,[11] as does one study that suggests that crenarchaeol may be produced by Marine Group II Euryarchaeota.[10]
TEX86 paleothermometer
The number of rings in GDGT hydrocarbon chains is temperature dependent and provides the basis for the TEX86 paleothermometer, a proxy for measuring ancient sea surface temperature (SST)[22] that relies on measurements of the abundances of crenarchaeol and its isomers. Crenarchaeol has a regioisomer which, based on radiocarbon analysis, may have a different origin than other isoprenoid GDGTs. Possible sources for the regioisomer include benthic archaea and diagensis of crenarchaeol, as the regioisomer is found in low abundance in surface waters and in cultures of pelagic thaumarchaea. Despite this, if it is excluded from TEX86 calculations, the paleothermometer's correlation with sea surface temperature becomes less apparent, indicating it is a necessary component of TEX86.[23]
Isolation and measurement
GDGTs such as crenarchaeol can be analyzed using high-performance liquid chromatography/atmospheric pressure chemical ionization-mass spectrometry (HPLC/APCI-MS) following extraction and acid hydrolysis.[24] Acid hydrolysis cleaves the polar head groups from the molecule, leaving the nonpolar chains behind. This is required for chromatography, which is not well suited to analysis of polar molecules. A variety of extraction techniques have been demonstrated to be effective for GDGTs. One common method is extraction by ultrasonication with methanol followed by washes of the nonpolar solvent dichloromethane (DCM).[24] GDGTs have characteristic [M + H]+ - 18 and [M + H]+ - 74 ions[24] that, for crenarchaeol, have masses of 1218 and 1162 Da, respectively. Relative abundances of GDGTs can be determined by integrating the peak areas of their characteristic ions.
References
- Pitcher A, Rychlik N, Hopmans EC, Spieck E, Rijpstra WI, Ossebaar J, Schouten S, Wagner M, Damsté JS (April 2010). "Crenarchaeol dominates the membrane lipids of Candidatus Nitrososphaera gargensis, a thermophilic group I.1b Archaeon". The ISME Journal. 4 (4): 542–52. doi:10.1038/ismej.2009.138. PMID 20033067.
- Jenkyns, H. C.; Schouten-Huibers, L.; Schouten, S.; Sinninghe Damsté, J. S. (2012-02-02). "Warm Middle Jurassic–Early Cretaceous high-latitude sea-surface temperatures from the Southern Ocean". Climate of the Past. 8 (1): 215–226. Bibcode:2012CliPa...8..215J. doi:10.5194/cp-8-215-2012. ISSN 1814-9332.
- Hoefs M, Schouten S, De Leeuw JW, King LL, Wakeham SG, Damste J (August 1997). "Ether lipids of planktonic archaea in the marine water column". Applied and Environmental Microbiology. 63 (8): 3090–5. PMC 1389224. PMID 16535669.
- Schouten S, Hopmans EC, Pancost RD, Damste JS (December 2000). "Widespread occurrence of structurally diverse tetraether membrane lipids: evidence for the ubiquitous presence of low-temperature relatives of hyperthermophiles". Proceedings of the National Academy of Sciences of the United States of America. 97 (26): 14421–6. doi:10.1073/pnas.97.26.14421. PMC 18934. PMID 11121044.
- DeLong EF, King LL, Massana R, Cittone H, Murray A, Schleper C, Wakeham SG (March 1998). "Dibiphytanyl ether lipids in nonthermophilic crenarchaeotes". Applied and Environmental Microbiology. 64 (3): 1133–8. PMC 106379. PMID 9501451.
- Damsté JS, Schouten S, Hopmans EC, van Duin AC, Geenevasen JA (October 2002). "Crenarchaeol: the characteristic core glycerol dibiphytanyl glycerol tetraether membrane lipid of cosmopolitan pelagic crenarchaeota". Journal of Lipid Research. 43 (10): 1641–51. doi:10.1194/jlr.M200148-JLR200. PMID 12364548.
- Brochier-Armanet C, Boussau B, Gribaldo S, Forterre P (March 2008). "Mesophilic Crenarchaeota: proposal for a third archaeal phylum, the Thaumarchaeota". Nature Reviews. Microbiology. 6 (3): 245–52. doi:10.1038/nrmicro1852. PMID 18274537.
- Schouten S, Hopmans EC, Baas M, Boumann H, Standfest S, Könneke M, Stahl DA, Sinninghe Damsté JS (April 2008). "Intact membrane lipids of "Candidatus Nitrosopumilus maritimus," a cultivated representative of the cosmopolitan mesophilic group I Crenarchaeota". Applied and Environmental Microbiology. 74 (8): 2433–40. doi:10.1128/AEM.01709-07. PMC 2293165. PMID 18296531.
- de la Torre JR, Walker CB, Ingalls AE, Könneke M, Stahl DA (March 2008). "Cultivation of a thermophilic ammonia oxidizing archaeon synthesizing crenarchaeol". Environmental Microbiology. 10 (3): 810–8. doi:10.1111/j.1462-2920.2007.01506.x. PMID 18205821.
- Lincoln SA, Wai B, Eppley JM, Church MJ, Summons RE, DeLong EF (July 2014). "Planktonic Euryarchaeota are a significant source of archaeal tetraether lipids in the ocean". Proceedings of the National Academy of Sciences of the United States of America. 111 (27): 9858–63. Bibcode:2014PNAS..111.9858L. doi:10.1073/pnas.1409439111. PMC 4103328. PMID 24946804.
- Rush D, Sinninghe Damsté JS (June 2017). "Lipids as paleomarkers to constrain the marine nitrogen cycle". Environmental Microbiology. 19 (6): 2119–2132. doi:10.1111/1462-2920.13682. PMC 5516240. PMID 28142226.
- Villanueva L, Schouten S, Damsté JS (January 2017). "Phylogenomic analysis of lipid biosynthetic genes of Archaea shed light on the 'lipid divide'". Environmental Microbiology. 19 (1): 54–69. doi:10.1111/1462-2920.13361. PMID 27112361.
- Schouten, Stefan; Hopmans, Ellen C.; Sinninghe Damsté, Jaap S. (2013-01-01). "The organic geochemistry of glycerol dialkyl glycerol tetraether lipids: A review". Organic Geochemistry. 54: 19–61. doi:10.1016/j.orggeochem.2012.09.006. ISSN 0146-6380.
- Langworthy TA, Holzer G, Zeikus JG, Tornabene TG (1983-01-01). "Iso- and Anteiso-Branched Glycerol Diethers of the Thermophilic Anaerobe Thermodesulfotobacterium commune". Systematic and Applied Microbiology. 4 (1): 1–17. doi:10.1016/S0723-2020(83)80029-0. PMID 23196295.
- Schouten, Stefan; Hopmans, Ellen C.; Schefuß, Enno; Sinninghe Damsté, Jaap S. (2002). "Distributional variations in marine crenarchaeotal membrane lipids: a new tool for reconstructing ancient sea water temperatures?". Earth and Planetary Science Letters. 204 (1–2): 265–274. Bibcode:2002E&PSL.204..265S. doi:10.1016/S0012-821X(02)00979-2.
- Schouten, Stefan; Hopmans, Ellen C.; Schefuß, Enno; Sinninghe Damsté, Jaap S. (2002-11-30). "Distributional variations in marine crenarchaeotal membrane lipids: a new tool for reconstructing ancient sea water temperatures?". Earth and Planetary Science Letters. 204 (1): 265–274. Bibcode:2002E&PSL.204..265S. doi:10.1016/S0012-821X(02)00979-2. ISSN 0012-821X.
- Mollenhauer, Gesine; Eglinton, Timothy I.; Hopmans, Ellen C.; Sinninghe Damsté, Jaap S. (2008-08-01). "A radiocarbon-based assessment of the preservation characteristics of crenarchaeol and alkenones from continental margin sediments" (PDF). Organic Geochemistry. Advances in Organic Geochemistry 2007. 39 (8): 1039–1045. doi:10.1016/j.orggeochem.2008.02.006. hdl:1912/2459. ISSN 0146-6380.
- Karner MB, DeLong EF, Karl DM (January 2001). "Archaeal dominance in the mesopelagic zone of the Pacific Ocean". Nature. 409 (6819): 507–10. Bibcode:2001Natur.409..507K. doi:10.1038/35054051. PMID 11206545.
- Könneke M, Bernhard AE, de la Torre JR, Walker CB, Waterbury JB, Stahl DA (September 2005). "Isolation of an autotrophic ammonia-oxidizing marine archaeon". Nature. 437 (7058): 543–6. Bibcode:2005Natur.437..543K. doi:10.1038/nature03911. PMID 16177789.
- Pitcher, Angela; Wuchter, Cornelia; Siedenberg, Kathi; Schouten, Stefan; Sinninghe Damsté, Jaap S. (2011). "Crenarchaeol tracks winter blooms of ammonia-oxidizing Thaumarchaeota in the coastal North Sea". Limnology and Oceanography. 56 (6): 2308–2318. Bibcode:2011LimOc..56.2308P. doi:10.4319/lo.2011.56.6.2308. ISSN 0024-3590.
- Mussmann M, Brito I, Pitcher A, Sinninghe Damsté JS, Hatzenpichler R, Richter A, Nielsen JL, Nielsen PH, Müller A, Daims H, Wagner M, Head IM (October 2011). "Thaumarchaeotes abundant in refinery nitrifying sludges express amoA but are not obligate autotrophic ammonia oxidizers". Proceedings of the National Academy of Sciences of the United States of America. 108 (40): 16771–6. Bibcode:2011PNAS..10816771M. doi:10.1073/pnas.1106427108. PMC 3189051. PMID 21930919.
- Kim, Jung-Hyun; van der Meer, Jaap; Schouten, Stefan; Helmke, Peer; Willmott, Veronica; Sangiorgi, Francesca; Koç, Nalân; Hopmans, Ellen C.; Damsté, Jaap S. Sinninghe (2010-08-15). "New indices and calibrations derived from the distribution of crenarchaeal isoprenoid tetraether lipids: Implications for past sea surface temperature reconstructions". Geochimica et Cosmochimica Acta. 74 (16): 4639–4654. Bibcode:2010GeCoA..74.4639K. doi:10.1016/j.gca.2010.05.027. ISSN 0016-7037.
- Shah, Sunita R.; Mollenhauer, Gesine; Ohkouchi, Naohiko; Eglinton, Timothy I.; Pearson, Ann (2008). "Origins of archaeal tetraether lipids in sediments: Insights from radiocarbon analysis". Geochimica et Cosmochimica Acta. 72 (18): 4577–4594. Bibcode:2008GeCoA..72.4577S. doi:10.1016/j.gca.2008.06.021. hdl:1912/2486.
- Schouten S, Huguet C, Hopmans EC, Kienhuis MV, Damsté JS (April 2007). "Analytical methodology for TEX86 paleothermometry by high-performance liquid chromatography/atmospheric pressure chemical ionization-mass spectrometry". Analytical Chemistry. 79 (7): 2940–4. doi:10.1021/ac062339v. PMID 17311408.