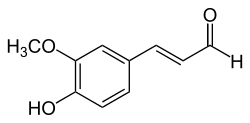
Coniferyl aldehyde
Coniferyl aldehyde is a low molecular weight phenolic compound susceptible to be extracted from cork stoppers into wine.[1]
 | |
| Names | |
|---|---|
| IUPAC names
(Z)-3-(4-hydroxy-3-methoxyphenyl)prop-2-enal (E)-3-(4-hydroxy-3-methoxyphenyl)prop-2-enal | |
| Other names
coniferaldehyde cis-coniferyl aldehyde trans-coniferyl aldehyde | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.006.618 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H10O3 | |
| Molar mass | 178.18 g/mol |
| Density | 1.186 g/mL |
| Melting point | 80 °C (176 °F; 353 K) |
| Boiling point | 338.8 °C (641.8 °F; 612.0 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Metabolism
Coniferyl-alcohol dehydrogenase uses coniferyl alcohol and NADP+ to produce coniferyl aldehyde, NADPH, and H+.
Coniferyl-aldehyde dehydrogenase uses coniferyl aldehyde, H2O, NAD+, and NADP+ to produce ferulate, NADH, NADPH, and H+.
Dihydroflavonol 4-reductase uses sinapaldehyde or coniferyl aldehyde or coumaraldehyde and NADPH to produce sinapyl alcohol or coniferyl alcohol or coumaryl alcohol respectively and NADP+.
gollark: I set it once on install and do not want to change it since it seems annoying.
gollark: Bold of you to assume I know how to do that and would be bothered.
gollark: The timestamps didn't match my timezone, if I remember right, thus not me.
gollark: Nonsense, it was obviously gibson.
gollark: And could at the time.
See also
- Phenolic compounds in wine
References
- Conde, Elvira; Cadahía, Estrella; García Vallejo, María Concepción; Fernández de Simón, Brígida (1998). "Polyphenolic Composition of Quercus suber Cork from Different Spanish Provenances". J. Agric. Food Chem. 46 (8): 3166–3171. doi:10.1021/jf970863k.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.