Choline chloride
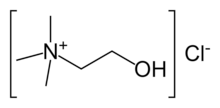
Choline chloride is an organic compound with the formula (CH3)3NCH2CH2OH]Cl. It is bifunctional, containing both quaternary ammonium salt and an alcohol. The cation is choline, which occurs naturally. It is a white, water-soluble salt used mainly in animal feed.[2]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
2-Hydroxy-N,N,N-trimethylethanaminium chloride OR (2-hydroxyethyl)trimethylammonium chloride | |
| Other names
hepacholine, biocolina and lipotril. | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.596 |
| E number | E1001(iii) (additional chemicals) |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H14ClNO | |
| Molar mass | 139.62 g·mol−1 |
| Appearance | White or deliquescent crystals |
| Melting point | 302 °C (576 °F; 575 K) (decomposes) |
| very soluble (>650 g/l)[1] | |
| Hazards | |
| Safety data sheet | External MSDS |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis
In the laboratory, choline can be prepared by methylation of dimethylethanolamine with methyl chloride.
Choline chloride is mass-produced with world production estimated at 160 000 tons in 1999.[2] Industrially, it is produce by the reaction of ethylene oxide, hydrogen chloride, and trimethylamine,[3] or from the pre-formed salt:[4]
Applications
It is an important additive in feed especially for chickens where it accelerates growth. It forms a deep eutectic solvent with urea, ethylene glycol, glycerol, and many other compounds.
It is also used as a clay control additive in fluids used for hydraulic fracturing.[5]
Related salts
Other commercial choline salts are choline hydroxide and choline bitartrate. In foodstuffs, the compound is often present as phosphatidylcholine.
References
- "Chemical Safety Information from Intergovernmental Organizations - Choline Chloride" (PDF). Archived from the original (PDF) on 2017-07-12.
- Matthias Frauenkron, Johann-Peter Melder, Günther Ruider, Roland Rossbacher, Hartmut Höke (2002). "Ethanolamines and Propanolamines". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a10_001.CS1 maint: uses authors parameter (link)
- |title= Johnson Matthey Process Technology - Choline chloride licensed process
- "Choline chloride" (PDF). Screening Information Data Set (SIDS) for High Production Volume Chemicals. IPCS INCHEM. Archived from the original (PDF) on 2017-07-12. Retrieved 2009-11-10.
- "What Chemicals Are Used". FracFocus. Retrieved 19 September 2014.
