Chlorographene
Chlorographene is fully chlorinated graphene with the chemical formula of (CCl)n.[1][2] Upon reaction with chlorine, graphene's sp2 planar lattice structure is transformed to sp3 hybridized buckled structure, this structure is similar to hydrogenated graphene (graphane) and fluorinated graphene (fluorographene).[3]
Early derivatives
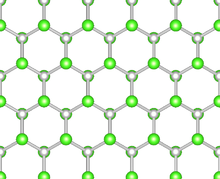
Although graphene is one of the most mechanically strong material having a wide range of extraordinary properties, practical device applications are limited by its metallic behavior and sensitivity to surface adsorbates. Efforts to synthesize chemically modified graphene composites with tailored electronic, optical, and chemical properties have presented new directions in graphene research. In particular, band gap engineering of graphene through chemical modification, such as oxygenation,[4] hydrogenation[5] and fluorination[6][7] is appealing for electronic applications, since the scalable fabrication of graphene-based devices without disturbing the strong honeycomb lattice has become possible. However, due to the complex atomic structure of grapheneoxides (GOs) and thermal instabilities of hydrogenated graphenes (CHs) even at low temperatures, search for the novel graphene-based materials is still continuing. Easy synthesis, high-quality insulating behavior and extraordinary mechanical strength of fluorographene (CF) have inspired intense research on other halogen decorated graphene derivatives.
Synthesis
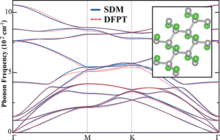
In addition to three known derivatives of graphene: grapheneoxide GO, graphane CH and fluorographene CF, the successful synthesis of chlorinated graphene (chlorographene) was also achieved very recently. It is experimentally demonstrated that nondestructive and patternable conversion of graphene is possible by using various photochemical chlorination techniques. Theoretical investigations have revealed that the covalently bonded chair conformation of chlorographene (formulated as CCl) is found to be stable even at room temperature.
Electronic properties

Chlorographene is a nonmagnetic semiconductor with 1.2 eV direct band gap. Top of the valence band and bottom of the conduction band locate at Gamma point (center of the Brillouin zone). Its electronic properties are more sensitive to applied strain than other graphene derivatives such as graphane and fluorographene.
References
- Sahin, H (2012). "Chlorine Adsorption on Graphene: Chlorographene". The Journal of Physical Chemistry C. 116 (45): 24075–24083. arXiv:1211.5242. doi:10.1021/jp307006c.
- Li, B (2011). "Photochemical Chlorination of Graphene". ACS Nano. 5 (7): 5957–61. doi:10.1021/nn201731t. PMID 21657242.
- Garcia, J. C.; de Lima, D. B.; Assali, L. V. C.; Justo, J. F. (2011). "Group IV graphene- and graphane-like nanosheets". J. Phys. Chem. C. 115 (27): 13242. arXiv:1204.2875. doi:10.1021/jp203657w.
- Chen, D (2012). "Graphene Oxide: Preparation, Functionalization, and Electrochemical Applications". Chemical Reviews. 112 (11): 6027–53. doi:10.1021/cr300115g. PMID 22889102.
- Sofo, Jorge O.; et al. (2007). "Graphane: A two-dimensional hydrocarbon". Physical Review B. 75 (15): 153401–4. arXiv:cond-mat/0606704. Bibcode:2007PhRvB..75o3401S. doi:10.1103/PhysRevB.75.153401.
- Properties of Fluorinated Graphene Films Jeremy T. Robinson; James S. Burgess; Chad E. Junkermeier; Stefan C. Badescu; Thomas L. Reinecke; F. Keith Perkins; Maxim K. Zalalutdniov; Jeffrey W. Baldwin; James C. Culbertson; Paul E. Sheehan; Eric S. Snow (2010). "Properties of Fluorinated Graphene Films". Nano Letters. 10 (8): 3001–3005. Bibcode:2010NanoL..10.3001R. doi:10.1021/nl101437p. PMID 20698613.
- Rahul R. Nair, Wencai Ren, Rashid Jalil, Ibtsam Riaz, Vasyl G. Kravets, Liam Britnell, Peter Blake, Fredrik Schedin, Alexander S. Mayorov, Shengjun Yuan, Mikhail I. Katsnelson, Hui-Ming Cheng, Wlodek Strupinski, Lyubov G. Bulusheva, Alexander V. Okotrub, Irina V. Grigorieva, Alexander N. Grigorenko, Kostya S. Novoselov, and Andre K. Geim (2010). "Fluorographene: A Two-Dimensional Counterpart of Teflon". Small. 6 (24): 2877–2884. arXiv:1006.3016. doi:10.1002/smll.201001555. PMID 21053339.CS1 maint: multiple names: authors list (link)
External links
| Look up chlorographene in Wiktionary, the free dictionary. |