Carbon nanohoop
Carbon nanohoops are a class of molecules consisting of aromatic sections curved out of planarity by the inherent cyclic geometry of the molecule. This class of molecules came into existence with the synthesis of cycloparaphenylenes[1] and since then has been expanded into cyclonaphthylenes,[2] cyclochrysenynlenes,[3] and even cyclohexabenzocoronenylenes.[4] Carbon nanohoops often map on to a certain chirality of carbon nanotube. If the diameter is adequate, these molecules can host a fullerene. For example, [10]cycloparaphenylene can host a C60 fullerene.
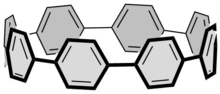
The first carbon nanohoop was entirely made of phenylenes. Newer examples contain other aromatic repeating units.
References
- Jasti, Ramesh; Bhattacharjee, Joydeep; Neaton, Jeffrey B.; Bertozzi, Carolyn R. (2008). "Synthesis, Characterization, and Theory of [9]-, [12]-, and [18]Cycloparaphenylene: Carbon Nanohoop Structures". Journal of the American Chemical Society. 130 (52): 17646–17647. doi:10.1021/ja807126u. PMC 2709987. PMID 19055403.
- Okada, Keishu; Yagi, Akiko; Segawa, Yasutomo; Itami, Kenichiro (2017). "Synthesis and properties of [8]-, [10]-, [12]-, and [16]cyclo-1,4-naphthylenes". Chemical Science. 8 (1): 661–667. doi:10.1039/C6SC04048A. PMC 5297897. PMID 28451214.
- Sun, Zhe; Suenaga, Takuya; Sarkar, Parantap; Sato, Sota; Kotani, Motoko; Isobe, Hiroyuki (2016). "Stereoisomerism, crystal structures, and dynamics of belt-shaped cyclonaphthylenes" (PDF). Proceedings of the National Academy of Sciences. 113 (29): 8109–8114. doi:10.1073/pnas.1606530113. PMC 4961134. PMID 27357686.
- Nakagawa, Yuta; Sekiguchi, Ryuta; Kawakami, Jun; Ito, Shunji (2019). "Preparation of a large-sized highly flexible carbon nanohoop". Organic & Biomolecular Chemistry. 17 (28): 6843–6853. doi:10.1039/C9OB00763F. PMID 31263811.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.