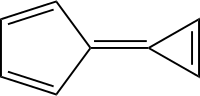
Calicene
Calicene or triapentafulvalene is a hydrocarbon of the fulvalene class with chemical formula C8H6, composed of linked a cyclopentadiene ring and a cyclopropene ring.
 | |
| Names | |
|---|---|
| IUPAC name
5-(2-Cyclopropen-1-ylidene)-1,3-cyclopentadiene | |
| Other names
Triapentafulvalene | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H6 | |
| Molar mass | 102.136 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Properties

Very high resonance energy is predicted by Hückel method,[1] however its resonance energy is not high. The central double bond is polarized with a partial positive charge on the carbon atom of triangular ring and a partial negative charge on the carbon atom of pentagonal ring, in keeping with added Hückel's rule stability of rings containing 2 π electrons and 6 π electrons respectively. Calicene's dipole moment has been computed to be 4.66 D.[2] Several compounds that contains two or more calicene subunits are aromatic, such as trans-bicalicene[2] (ring compound) or poly-2,7-[N]calicenes (chain compound)[3]
Despite several attempts to prepare it, the parent calicene has so far defied attempts at synthesis.[4] However, 1,2,3,4,5,6-hexaphenylcalicene has been prepared and an experimental dipole moment of 6.3 D was measured.[5]
References
- Schaad, L. J.; B. Andes Hess , Jr (2001). "Dewar Resonance Energy". Chemical Reviews. 101 (5): 1465–1476. doi:10.1021/cr9903609.
- Oziminski, W. P.; M. Palusia (2013). "Capturing the elusive aromaticity of bicalicene". Physical Chemistry Chemical Physics. 15 (9): 3286–3293. Bibcode:2013PCCP...15.3286O. doi:10.1039/C2CP43426A. PMID 23358331.
- Ratanadachanakin, Thawalrat; Collier, Willard E. r (2015). "Aromaticity of a series of poly-2,7-[N]calicenes" (PDF). Maejo International Journal of Science and Technology. 9 (1): 21–31.
- de Meijere, Armin (2014). Houben-Weyl Methods of Organic Chemistry Vol. E 17d, 4th Edition Supplement: Carbocyclic Three-Membered Ring Compounds, Cyclopropenes, Author Index, Compound Index. Stuttgart: Georg Thieme Verlag. p. 2967. ISBN 978-3131819741.
- Agranat, Israel; Bergmann, Ernst D. (1965-01-01). "Hexaphenyltriapentafulvalene". Chemical Communications (London). 0 (21): 512–513. doi:10.1039/C19650000512. ISSN 0009-241X.