CIDNP
Discovered in 1967 by Bargon et al.; Ward and Lowler, CIDNP (chemically induced dynamic nuclear polarization), often pronounced like "kidnip", is an nuclear magnetic resonance (NMR) technique that is used to study chemical reactions that involve radicals. It is related to chemically induced dynamic electron polarization (CIDEP) insofar as the radical-pair mechanism explains both phenomena.[1]
Concept and experimental set-up
The effect is detected by NMR spectroscopy, usually using 1H NMR spectrum, as enhanced absorption or emission signals ("negative peaks"). The effect arises when unpaired electrons (radicals) are generated during a chemical reaction. Because the magnetic moment of an electron is more than 600x that of a proton, the spins of many protons are polarized beyond the usual thermal Boltzmann distribution.
The CIDNP experiment is conducted within the NMR tube. The radicals are produced by thermal or photochemical reactions, usually from colligation and diffusion, or disproportionation of radical pairs.
Radical pair mechanism
The generation of CIDNP in a typical photochemical system (target + photosensitizer, flavin in this example) is a cyclic photochemical process shown schematically in Figure 1. The chain of reactions is initiated by a blue light photon, which excites the flavin mononucleotide (FMN) photosensitizer to the singlet excited state. The fluorescence quantum yield of this state is rather low, and approximately half of the molecules undergo intersystem crossing into the long-lived triplet state. Triplet FMN has a remarkable electron affinity. If a molecule with a low ionization potential (e.g. phenols, polyaromatics) is present in the system, the diffusion-limited electron transfer reaction forms a spin-correlated triplet electron transfer state – a radical pair. The kinetics are complicated and may involve multiple protonations and deprotonations, and hence exhibit pH dependence.
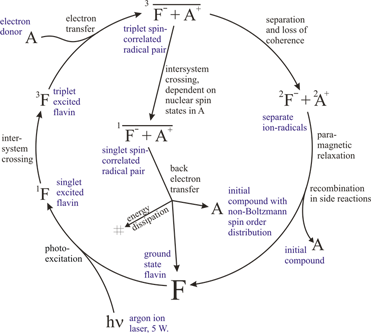
The radical pair may either cross over to a singlet electron state and then recombine, or separate and perish in side reactions. The relative probability of these two pathways for a given radical pair depends on the nuclear spin state and leads to the nuclear spin state sorting and observable nuclear polarization.
Applications
Detected as enhanced absorptive or emissive signals in the NMR spectra of the reaction products, CIDNP has been exploited for the last 30 years to characterise transient free radicals and their reaction mechanisms. In certain cases, CIDNP also offers the possibility of large improvements in NMR sensitivity. The principal application of this photo-CIDNP technique, as devised by Kaptein in 1978, has been to proteins in which the aromatic amino acid residues histidine, tryptophan and tyrosine can be polarized using flavins or other aza-aromatics as photosensitisers. The key feature of the method is that only solvent accessible histidine, tryptophan and tyrosine residues can undergo the radical pair reactions that result in nuclear polarization. Photo-CIDNP has thus been used to probe the surface structure of proteins, both in native and partially folded states, and their interactions with molecules that modify the accessibility of the reactive side chains.
Although usually observed in liquids, the photo-CIDNP effect has also been detected in solid state, for example on 13C and 15N nuclei in photosynthetic reaction centres, where significant nuclear polarization can accumulate as a result of spin selection processes in the electron transfer reactions.
History
CIDNP was discovered in 1967 by Bargon and Fischer, and, independently, by Ward and Lawler. Early theories were based on dynamic nuclear polarisation (hence the name). The subsequent experiments, however, have found that in many cases DNP fails to explain CIDNP polarization phase. In 1969 an alternative explanation was proposed by Closs, and, independently, by Kaptein and Oosterhoff, which relied on the ability of nuclear spin interactions to alter the recombination probability in reactions that proceed through radical pairs. This mechanism, known as the radical pair mechanism is currently accepted as the most common cause of CIDNP. There are, however, exceptions, and the DNP mechanism was found to be operational, for example, in many fluorine-containing radicals.
See also
- Dynamic nuclear polarisation
- Electron paramagnetic resonance
References
- Vyushkova, Maria (April 2011). "Basic principles and applications of spin chemistry" (PDF). nd.edu. University of Notre Dame. Retrieved November 21, 2016.
- L.T. Muus, P.W. Atkins, K.A. McLauchlan, J.B. Pedersen (ed.), Chemically induced magnetic polarisation, D. Reidel, Dordrecht, 1977.
- M. Goez, Photochemically induced dynamic nuclear polarization, Adv. Photochem. 23 (1997) 63-163.
- R. Kaptein, Photo-CIDNP studies of proteins, Biol. Magn. Res. 4 (1982) 145-191.
- R. Kaptein, K. Dijkstra, K. Nicolay, Laser photo-CIDNP as a surface probe for proteins in solution, Nature 274 (1978) 293-294.
- P.J. Hore, R.W. Broadhurst, Progr. NMR Spec. 25 (1993) 345-402. Abstract
- I. Kuprov, P.J. Hore, J. Magn. Res. 168 (2004) 1-7 Article
- S. Prakash et al., J. Am. Chem. Soc. 128 (2006) 12794-12799 Article