Brassinosteroid insensitive-1
Brassinosteroid insensitive 1 (BRI1) is the major receptor of the plant hormone brassinosteroid.[1][2] It plays very important roles in plant development, especially in the control of cell elongation and for the tolerance of environmental stresses. BRI1 enhances cell elongation,[1] promotes pollen development,[3] controls vasculature development[2] and promotes chilling and freezing tolerance.[4] BRI1 is one of the most well studied hormone receptors and it acts a model for the study of membrane-bound receptors in plants.
| Brassinosteroid insensitive 1 | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Organism | |||||||
| Symbol | BRI1 | ||||||
| Entrez | 830095 | ||||||
| HomoloGene | 91895 | ||||||
| RefSeq (mRNA) | NM_120100.3 | ||||||
| RefSeq (Prot) | NP_195650.1 | ||||||
| UniProt | O22476 | ||||||
| Other data | |||||||
| EC number | 2.7.11.1 | ||||||
| Chromosome | 4: 18.32 - 18.33 Mb | ||||||
| |||||||
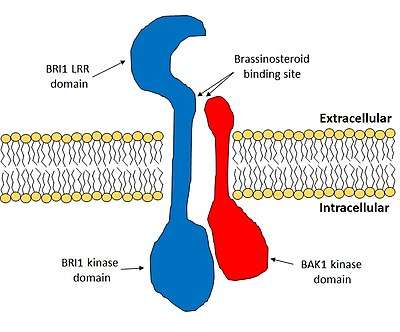
Structure
BRI1 is an integral membrane protein. On the extracellular side of the membrane lies a series of 25 leucine-rich repeats (LRRs). The LRR domain forms a horseshoe shape. An atypical LRR within this domain acts as the brassinosteroid binding site. Next to the LRR domain there is a single-pass transmembrane section. The intracelluar domain of BRI1 functions as a kinase and it is this domain triggers the phosphorylation cascade that results in changes of gene expression.[5]
Activation
In the absence of brassinosteroid, BRI1 is held in an inactive state by another protein, BRI1 kinase inhibitor 1 (BKI1).[6] When brassinosteroid binds to BRI1, it reduces the stability of the BRI1:BKI1 complex and promotes the binding of BRI1 to another membrane protein, BRI1-associated receptor kinase 1 (BAK1).[7] In the BRI1:BAK1 complex, both BRI1 and BAK1 make contact with the brassinosteroid molecule and for this reason they are considered a co-receptor.[5][7] BRI1 and BAK1 sequentially phosphorylate each other in their kinase domains, which results in the activation of BRI1. The activated kinase domain of BRI1 phosphorylates several receptor-like cytoplasmic kinases (RLCKs), notably the brassinosteroid signalling kinase (BSK) and constituitive differential growth 1 (CDG1) families. The RLCKs traduce this signal to downstream components, which ultimately results in the activation or de-activation of several transcription factors.[5]
Related proteins
BRI1-family proteins
In the model plan species Arabidopsis thaliana, BRI1 acts alongside two homologous proteins, known as BRI1-LIKE1 (BRL1) and BRL3.[2] The function of BRL1 and BRL3 appears to be restricted to the development of the vasculature system, but even in this context, BRI1 plays a more dominant role.[2] Both BRL1 and BRL3 are able to bind brassinosteroids and act as receptors.[2][5] A fourth BRI1-family protein, BRL2 cannot bind brassinosteroid and its function is unknown.[5]
FLS2
BRI1 belongs to the large leucine-rich receptor-like protein kinase family. There are many other members of this family of proteins,[8] and one of the most important is FSL2.[9] FLS2 acts as a detector of the bacterial protein flagellin and is important for plant immunity.[9] Surprisingly (given there different functions) the signal cascades of BRI1 and FLS2 share many of the same components.[10] Recently it was suggested that BRI1 and FLS2 localize to different 'nano-domains' on the cell membrane and it is this spatial separation that accounts for there very different signal outputs.[11]
References
- Wang ZY, Seto H, Fujioka S, Yoshida S, Chory J (March 2001). "BRI1 is a critical component of a plasma-membrane receptor for plant steroids". Nature. 410 (6826): 380–3. doi:10.1038/35066597. PMID 11268216.
- Caño-Delgado A, Yin Y, Yu C, Vafeados D, Mora-García S, Cheng JC, Nam KH, Li J, Chory J (November 2004). "BRL1 and BRL3 are novel brassinosteroid receptors that function in vascular differentiation in Arabidopsis". Development. 131 (21): 5341–51. doi:10.1242/dev.01403. PMID 15486337.
- Ye Q, Zhu W, Li L, Zhang S, Yin Y, Ma H, Wang X (March 2010). "Brassinosteroids control male fertility by regulating the expression of key genes involved in Arabidopsis anther and pollen development". Proceedings of the National Academy of Sciences of the United States of America. 107 (13): 6100–5. Bibcode:2010PNAS..107.6100Y. doi:10.1073/pnas.0912333107. PMC 2851861. PMID 20231470.
- Eremina M, Unterholzner SJ, Rathnayake AI, Castellanos M, Khan M, Kugler KG, May ST, Mayer KF, Rozhon W, Poppenberger B (October 2016). "Brassinosteroids participate in the control of basal and acquired freezing tolerance of plants". Proceedings of the National Academy of Sciences of the United States of America. 113 (40): E5982–E5991. doi:10.1073/pnas.1611477113. PMC 5056081. PMID 27655893.
- Belkhadir Y, Jaillais Y (April 2015). "The molecular circuitry of brassinosteroid signaling". The New Phytologist. 206 (2): 522–40. doi:10.1111/nph.13269. PMID 25615890.
- Wang J, Jiang J, Wang J, Chen L, Fan SL, Wu JW, Wang X, Wang ZX (November 2014). "Structural insights into the negative regulation of BRI1 signaling by BRI1-interacting protein BKI1". Cell Research. 24 (11): 1328–41. doi:10.1038/cr.2014.132. PMC 4220157. PMID 25331450.
- Nam KH, Li J (July 2002). "BRI1/BAK1, a receptor kinase pair mediating brassinosteroid signaling". Cell. 110 (2): 203–12. doi:10.1016/s0092-8674(02)00814-0. PMID 12150928.
- Smakowska-Luzan E, Mott GA, Parys K, Stegmann M, Howton TC, Layeghifard M, et al. (January 2018). "An extracellular network of Arabidopsis leucine-rich repeat receptor kinases". Nature. 553 (7688): 342–346. Bibcode:2018Natur.553..342S. doi:10.1038/nature25184. PMC 6485605. PMID 29320478.
- Gómez-Gómez L, Boller T (June 2000). "FLS2: an LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis". Molecular Cell. 5 (6): 1003–11. doi:10.1016/S1097-2765(00)80265-8. PMID 10911994.
- Chinchilla D, Zipfel C, Robatzek S, Kemmerling B, Nürnberger T, Jones JD, Felix G, Boller T (July 2007). "A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence". Nature. 448 (7152): 497–500. Bibcode:2007Natur.448..497C. doi:10.1038/nature05999. hdl:11858/00-001M-0000-0012-3840-F. PMID 17625569.
- Bücherl CA, Jarsch IK, Schudoma C, Segonzac C, Mbengue M, Robatzek S, MacLean D, Ott T, Zipfel C (March 2017). "Plant immune and growth receptors share common signalling components but localise to distinct plasma membrane nanodomains". eLife. 6. doi:10.7554/eLife.25114. PMC 5383397. PMID 28262094.