Bouveault aldehyde synthesis
The Bouveault aldehyde synthesis (also known as the Bouveault reaction) is a one-pot substitution reaction that replaces an alkyl or aryl halide with a formyl group using a N,N-disubstituted formamide.[1][2] For primary alkyl halides this produces the homologous aldehyde one carbon longer. For aryl halides this produces the corresponding carbaldehyde. The Bouveault aldehyde synthesis is an example of a formylation reaction, and is named for French scientist Louis Bouveault.
| Bouveault aldehyde synthesis | |
|---|---|
| Named after | Louis Bouveault |
| Reaction type | Carbon-carbon bond forming reaction |
| Identifiers | |
| RSC ontology ID | RXNO:0000533 |
Reaction mechanism
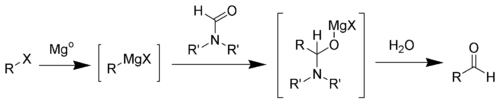
The first step of the Bouveault aldehyde synthesis is the formation of the Grignard reagent. Upon addition of a N,N-disubstituted formamide (such as dimethylformamide) a hemiaminal is formed, which can easily be hydrolyzed into the desired aldehyde.
Variations
Variants using organolithium reagents instead of magnesium-based Grignard reagents are also considered Bouveault aldehyde syntheses.[3]
See also
- Bodroux-Chichibabin aldehyde synthesis
- Bouveault–Blanc reduction
- Duff reaction
References
- Louis Bouveault (1904). "Modes de formation et de préparation des aldéhydes saturées de la série grasse" [Methods of preparation of saturated aldehydes of the aliphatic series]. Bull. Soc. Chim. Fr. (in French). 31: 1306–1322.
- Louis Bouveault (1904). "Nouvelle méthode générale synthétique de préparation des aldéhydes" [Novel general synthetic method for preparing aldehydes]. Bull. Soc. Chim. Fr. (in French). 31: 1322–1327.
- Jie Jack Li. Name Reactions: A Collection of Detailed Reaction Mechanisms. Springer, 2003. ISBN 3-540-40203-9
- Smith, L. I.; Nichols, J. (1941). "The Synthesis of Aldehydes from Grignard Reagents. II. Polymethylbenzaldehydes". J. Org. Chem. 6 (4): 489. doi:10.1021/jo01204a003.
- Sice, Jean (1953). "Preparation and Reactions of 2-Methoxythiophene". J. Am. Chem. Soc. 75 (15): 3697. doi:10.1021/ja01111a027.
- Jones, E. R. H. (1958). "210. Researches on acetylenic compounds. Part LX. The synthesis of three natural polyacetylenic hydrocarbons". J. Chem. Soc.: 1054. doi:10.1039/jr9580001054.