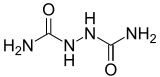
Biurea
Biurea is a chemical compound with the molecular formula C2H6N4O2. It is produced in food products containing azodicarbonamide, a common ingredient in bread flour, when they are cooked.[2] Upon exposure, biurea is rapidly eliminated from the body through excretion.[3]
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Hydrazine-1,2-dicarboxamide | |
| Systematic IUPAC name
(Carbamoylamino)urea[1] | |
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.003.408 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C2H6N4O2 | |
| Molar mass | 118.096 g·mol−1 |
| Appearance | White crystals |
| Thermochemistry | |
Std enthalpy of formation (ΔfH⦵298) |
−499.9–−497.5 kJ mol−1 |
Std enthalpy of combustion (ΔcH⦵298) |
−1.1471–−1.1447 MJ mol−1 |
| Related compounds | |
Related compounds |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Biurea is produced from urea and hydrazine by transamidation. Its major use is as a chemical intermediate in the production of azodicarbonamide, a common blowing agent.[4]
References
- "Biurea - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 26 March 2005. Identification. Retrieved 27 June 2012.
- Azodicarbonamide, FAO Nutrition Meetings, Report Series No. 40A,B,C
- Mewhinney, JA; Ayres, PH; Bechtold, WE; Dutcher, JS; Cheng, YS; Bond, JA; Medinsky, MA; Henderson, RF; Birnbaum, LS (1987). "The fate of inhaled azodicarbonamide in rats" (PDF). Fundamental and Applied Toxicology. 8 (3): 372–81. doi:10.1016/0272-0590(87)90086-8. PMID 3569707.
- Eugene F. Rothgery (2004). "Hydrazine and Its Derivatives". Kirk-Othmer Encyclopedia of Chemical Technology. John Wiley and Sons. doi:10.1002/0471238961.0825041819030809.a01.pub2.
External links
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.