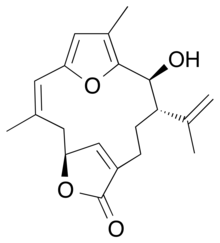
Bipinnatin J
Bipinnatin J is a diterpene isolated from the bipinnate sea plume Antillogorgia bipinnata, a sea fan found in the eastern Caribbean Sea.[1] It is one of the structurally simplest of the furanocembrenolides, and is speculated to be a biosynthetic precursor to a wide array cembrenolides along with the dehydroxylated analog, rubifolide.[2]
 | |
| Names | |
|---|---|
| IUPAC name
(2Z,5S,11S,12S)-12-Hydroxy-11-isopropenyl-3,14-dimethyl-6,16-dioxatricyclo[11.2.1.15,8]heptadeca-1(15),2,8(17),13-tetraen-7-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C20H24O4 | |
| Molar mass | 328.40216 |
| Melting point | 140 to 142 °C (284 to 288 °F; 413 to 415 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Biosynthesis
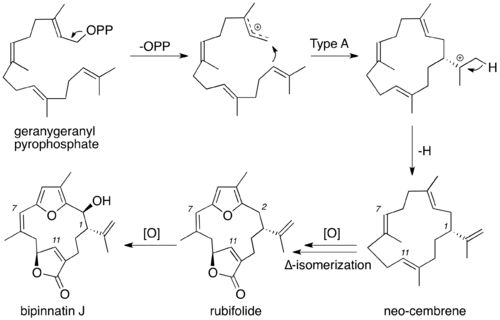
Although the exact biosynthesis of bipinnatin J has not been formally studied, the biosynthesis the core cembrane skeleton, neo-cembrene, has been extensively studied. Starting from geranylgeranyl pyrophosphate, the pyrophosphate leaves, creating the allyl carbocation. A type A cyclization then yields the 14-membered cembrane ring with the isopropyl cation outside the ring. Proton elimination then yields neo-cembrene.[3] From this point, the biosynthesis of bipinnatin J is speculative. Oxidation, most likely utilizing P450 monooxygenases, followed by ring closure creates both the furan and butenolide within the 14-membered ring. ∆ double bond isomerization of the C7-C8 olefin then occurs to afford the Z conformation, yielding rubifolide. Another oxidation of C2 then yields bipinnatin J.[2]
References
- Rodriguez, A. D.; Shi, J.-G. (1998). "The First Cembrane-Pseudopterane Cycloisomerization". J. Org. Chem. 63 (2): 420–421. doi:10.1021/jo971884g.
- Roethle, P. A.; Trauner, D. (2008). "The chemistry of marine furanocembranoids, pseudopteranes, gersolanes, and related natural products". Nat. Prod. Rep. 25 (2): 298–317. doi:10.1039/b705660p. PMID 18389139.
- MacMillan, J.; Beale, M. H. (1999). "Diterpene Biosynthesis". Comprehensive Natural Products Chemistry. 2: 217–243.