Bial's test
Bial's test is a chemical test for the presence of pentoses. It is named after Manfred Bial, a German physician. The components include orcinol, hydrochloric acid, and ferric chloride. A pentose, if present, will be dehydrated to form furfural which then reacts with the orcinol to generate a colored substance. The solution will turn bluish and a precipitate may form. The solution shows two absorption bands, one in the red between Fraunhofer lines B and C and the other near the D line.[1] An estimate of the relevant wavelengths can be made by referring to the Fraunhofer lines article.
| Classification | Colorimetric method |
|---|---|
| Analytes | Pentoses |
Composition
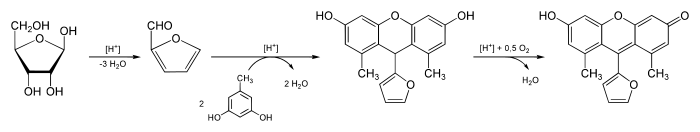
Bial's reagent consists of 0.4 g orcinol, 200 ml of concentrated hydrochloric acid and 0.5 ml of a 10% solution of ferric chloride.[2] Bial's test is used to distinguish pentoses from hexoses; this distinction is based on the color that develops in the presence of orcinol and iron (III) chloride. Furfural from pentoses gives a blue or green color. The related hydroxymethylfurfural from hexoses may give a muddy-brown, yellow or gray solution, but this is easily distinguishable from the green color of pentoses.
Quantitative version
The test may be performed as a quantitative colorimetric test using a spectrophotometer. Fernell and King have published a procedure for simultaneous determination of pentoses and hexoses from measurements at two wavelengths.[3] Various versions of this test are widely used for a quick chemical determination of RNA; in this context it is usually called the orcinol test.[4]
References
- Baldwin, E. and Bell, D.J., Cole's Practical Physiological Chemistry, published by Heffer, Cambridge, 1955, page 189
- Baldwin and Bell, page 189
- W. R. Fernell and H. K. King, The simultaneous determination of pentose and hexose in mixtures of sugars. Analyst, 1953,78, 80–83
- R.S. Hanson and J.A. Phillips, Chemical composition; in Gerhardt, Phillip, ed Manual of methods for General Bacteriology, American Society for Microblogy 1981, p. 349.