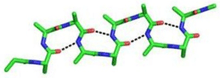
Beta bend ribbon
The beta bend ribbon, or beta-bend ribbon, is a structural feature in polypeptides[1][2][3][4][5][6][7] and proteins.[8] The shortest possible has six amino acid residues (numbered i to i+5) arranged as two overlapping hydrogen bonded beta turns in which the carbonyl group of residue i is hydrogen-bonded to the NH of residue i+3 while the carbonyl group of residue i+2 is hydrogen-bonded to the NH of residue i+5. In longer ribbons, this bonding is continued in peptides of 8, 10, etc., amino acid residues. A beta bend ribbon can be regarded as an aberrant 310 helix (3/10-helix) that has lost some of its hydrogen bonds.[9] Two websites are available to facilitate finding and examining these features in proteins: Motivated Proteins;[10] and PDBeMotif.[11]
The four main types of hydrogen-bonded beta turns are types I, I’, II and II’.[12] Beta bend ribbons may be formed from any of these types but type I is the commonest in proteins, as it is for single beta turns. Beta bend ribbons made from type I or I’ turns are somewhat twisted, while beta bend ribbons made from type II or II’ beta turns are flat. Beta bend ribbons with mixtures of different beta turn types also occur.
Type I beta-bend ribbons regularly occur in leucine-rich repeats, in the environments sometimes occupied by helices. A protein with a stack of these features is the extracellular ligand-binding domain of the Nogo receptor.[13] Another beta bend ribbon occurs in the GTPase-activating protein for Rho in the active, but not the inactive, form of the enzyme. The beta bend ribbon, which incorporates the catalytic arginine, allows its side-chain guanidino group to approach the active site and enhance enzyme activity.[14]
Polypeptides consisting of repeats of the dipeptide (α-amino-γ-lactam plus a conventional amino acid) have been shown to adopt a beta bend ribbon conformation.[15]
References
- Karle IL, Flippen-Anderson J, Sukumar M, Balaram P. Conformation of a 16-residue zervamicin IIA analog peptide containing three different structural features: 3(10)-helix, alpha-helix, and beta-bend ribbon. Proc Natl Acad Sci USA 1987;84:5087–5091
- Crisma M, Formaggio F, Moretto A, Toniolo C. Peptide helices based on alpha-amino acids. Biopolymers 2006;84:3–12
- Gupta M, Chauhan VS. De novo design of alpha,beta-didehydrophenylalanine-containing peptides. From models to applications. Biopolymers 2011;95:161–173
- Di Blasio B, Pavone V, Saviano PM, Lombardi A, Nastri F, Pedone C, Benedetti E, Crisma M, Anzolin M, Toniolo C. Structural characterization of the beta-bend ribbon spiral: crystallographic analysis of two long (L-Pro-Aib), sequential peptides. J Am Chem Soc 1991;114:6278–6291
- Madalengoita JS. A novel peptide fold: a repeating betaII-turn secondary structure. J Am Chem Soc 2000;122:4986–4987
- Formaggio F, Toniolo C. Electronic and vibrational signatures of peptide helical structures. A tribute to Anton Mario Tamburro. Chirality 2010;22:E30–E39
- Kennedy DF, Crisma M, Toniolo C, Chapman D. Studies of peptides forming 3/10- and alpha-helices and beta-bend ribbon structures in organic solution and in model membranes by Fourier Transform Infrared spectroscopy. Biochemistry 1991;30:6541–6548
- Hayward, SJ, Leader, DP, Al-Shubailly, F, Milner-White, EJ. (2014) Rings and ribbons in protein structures: Characterization using helical parameters and Ramachandran plots for repeating dipeptides. Proteins 2014; 82:230–239
- Toniolo C, Benedetti E (1991) The polypeptide 3/10-helix. Trends Biochem Sci 16: 350-353
- Leader DP, Milner-White, EJ (2009) Motivated Proteins: A web application for studying small three-dimensional protein motifs. BMC Bioinformatics 10:60
- Golovin A; Henrick K (2008) MSDmotif: exploring protein sites and motifs. BMC Bioinformatics 9:312
- Venkatachalam CM (1968) Stereochemical criteria for polypeptides and proteins V. Conformation of a system of three-linked peptide units. Biopolymers 6:1425-1436
- He XL, Bazan JF, McDermott G, Park JB, Wang K, Tessier-Lavigne M, He Z, Garcia KC. Structure of the Nogo receptor ectodomain: a recognition module implicated in myelin inhibition. Neuron 2003; 38:177–185.
- Rittinger K, Walker PA, Eccleston JF, Smerdon SJ, Gamblin SJ. Structure at 1.65 Å of RhoA and its GTPase-activating protein in complex with a transition-state analogue. Nature 1997;389:758–762.
- Martin V, Legrand B, Vezenkov LL. Turning peptide sequences into ribbon foldamers by a straightforward multicyclization reaction. Angewandte Chemie 2015;54:1-6.