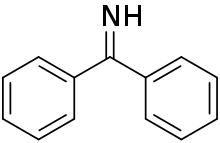
Benzophenone imine
Benzophenone imine is an organic compound with the formula of (C6H5)2C=NH. Benzophenone imine is widely used as a reagent for the protection of primary amines, and the starting materials to synthesize aniline.[1]
 | |
| Names | |
|---|---|
| IUPAC name
Diphenylmethanimine | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.103.715 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C13H11N | |
| Molar mass | 181.238 g·mol−1 |
| Hazards | |
| GHS pictograms |  |
| GHS Signal word | Warning |
GHS hazard statements |
H315, H319, H335 |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis
Benzophenone imine is available via the addition of MeOH to the complex of a nitrile and a Grignard reagent or the reaction between benzophenone and ammonia.
Synthesis by Grignard-nitrile complexes
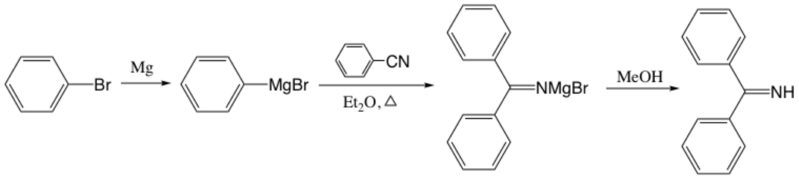
The first report about the preparation of ketimines involved Grignard-nitrile complexes followed by careful hydrolysis, which is known as Moureu-Mignonac ketimine synthesis.[3] Then Pickard and Tolbert improved the preparation by using methanol in the addition of Grignard-nitrile complexes.[2]
Synthesis by benzophenone and ammonia
In 1988, A. G. Guimanini discovered a new route to synthesize benzophenone imine by a reaction using benzophenone and ammonia. A chemical pure-grade ammonia gas is added to a benzophenone solution, forming Ph2C=NH2+. After sodium hydroxide pellets are added to the solution, the Ph2C=NH2+ is neutralized, generating the expected benzophenone imine.[4]
Applications
Protecting group for primary amines
Primary amines can be protected benzophenone imine, and the protected amines are stable in flash chromatography.[5]
Amination of aryl halides
Buchwald-Hartwig amination is a very important kind of reaction for coupling aromatic halide and amine to form carbon-nitrogen bonds with the help of palladium-involved catalysts. In order to obtain anilines, ammonia is required in this reaction. However, ammonia can bind to palladium tightly, which makes the normal Buchwald-Hartwig reaction unavailable. In 1997, Buchwald et. al found that benzophenone imine can be used as an ammonia-equivalent and solve the above limitations.[1]
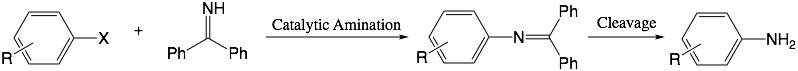
References
- Wolfe, John P.; Åhman, Jens; Sadighi, Joseph P.; Singer, Robert A.; Buchwald, Stephen L. (1997-09-08). "An Ammonia Equivalent for the Palladium-Catalyzed Amination of Aryl Halides and Triflates". Tetrahedron Letters. 38 (36): 6367–6370. doi:10.1016/S0040-4039(97)01465-2. ISSN 0040-4039.
- Pickard, P. L.; Tolbert, T. L. (December 1961). "An Improved Method of Ketimine Synthesis". The Journal of Organic Chemistry. 26 (12): 4886–4888. doi:10.1021/jo01070a025. ISSN 0022-3263.
- Moureau-Mignonac Ketimine Synthesis. Comprehensive Organic Name Reactions and Reagents. Hoboken, NJ, USA: John Wiley & Sons, Inc. 2010-09-15. pp. 1988–1990. doi:10.1002/9780470638859.conrr446. ISBN 9780470638859.
- Verardo, G.; Giumanini, A. G.; Strazzolini, P.; Poiana, M. (1988). "Ketimines From Ketones and Ammonia". Synthetic Communications. 18 (13): 1501–1511. doi:10.1080/00397918808081307.
- O'Donnell, Martin J. (2001-04-15). Benzophenone Imine. Encyclopedia of Reagents for Organic Synthesis. Chichester, UK: John Wiley & Sons, Ltd. doi:10.1002/047084289x.rb031. ISBN 978-0471936237.