Barbara Low (biochemist)
Barbara Wharton Low (March 23, 1920 – January 10, 2019) was a biochemist, biophysicist, and a researcher involved in discovering the structure of penicillin and the characteristics of other antibiotics. Her early work at Oxford University with Dorothy Hodgkin used X-ray crystallography to confirm the molecular structure of penicillin, which at the time was the largest molecule whose structure has been determined using that method. Later graduate work saw her study with Linus Pauling and Edwin Cohn before becoming a professor in her own right. Low's laboratory would accomplish the discovery of the pi helix, investigate the structure of insulin, and conduct research into neurotoxins.
Barbara Low | |
|---|---|
 Low in 1960 | |
| Born | Barbara Wharton Low March 23, 1920 Lancaster, England |
| Died | January 10, 2019 (aged 98)[1] Riverdale, Bronx, New York, US |
| Nationality | British/American |
| Education | B.A. (1943), M.A. (1946), D.Phil. (1948) |
| Alma mater | Somerville College, Oxford |
| Known for | Discovery of penicillin structure, discovery of pi helix |
| Title | Professor of Biochemistry & Molecular Biophysics |
| Partner(s) | Metchie J. E. Budka |
| Scientific career | |
| Fields | Biochemistry, Biophysics |
| Institutions | Columbia University |
| Doctoral advisor | Dorothy Hodgkin |
| Notable students | Frederic M. Richards, Helen M. Berman, Clara Shoemaker |
Childhood and education
Low was born on March 23, 1920 in Lancaster, England to her parents, Matthew Low and Mary Jane Wharton. She undertook her tertiary education at Somerville College, Oxford, graduating with a bachelor's degree in 1943. That same year she began work under the biochemist Dorothy Hodgkin as a research assistant for the university's department of chemical crystallography.[2] Due to Hodgkin's focus on protein crystallography, for which she was later awarded a Nobel prize, Low and her academic colleagues were engaged in researching the use of X-rays to determine the structure of crystallized proteins.[3] Low obtained Master's and doctoral degrees in chemistry from Oxford University in 1946 and 1948 respectively, before moving on to a research associate position at the California Institute of Technology (Caltech). To do so, she obtained a United States passport through emigration and later full citizenship in 1956.[1]
While at Caltech, she worked under Nobel Laureate Linus Pauling for a year before moving to another yearly research associate position at Harvard University working with Edwin Cohn.[1] The following year, in 1950, Harvard offered Low her first academic appointment as assistant professor of biophysical chemistry. She relocated to Columbia University in 1956 as an associate professor, and was promoted to a full professorship in 1966. Low continued at Columbia until her retirement in 1990 as professor emerita of biochemistry and molecular biophysics. She still did routine academic rounds as a "Special Lecturer" at the university, however, up until 2013.[1][3]
Career
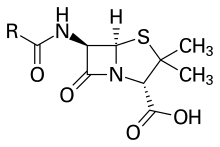
During Low's early work with the Hodgkin laboratory in the final years of World War II, she discovered the sulfur elemental components of penicillin that allowed for its mass production and later transformation into other antibiotic compounds. Up to that point, a pure sample of penicillin had not been successfully synthesized due to a lack of understanding of its physical structure of the compound, specifically the variation of its penam core.[3] Due to the size of the molecule, only careful examination of the X-ray results allowed for any information on the overall construction, but the two finally completed the investigation in 1945.[4] During this time, she was one of the first scientists in the United States to conduct studies of X-ray diffraction of crystalline proteins in a laboratory setting.[5] At that time, it was the largest molecule ever to have had its structure determined by crystallography.[4] Because the knowledge Low and Hodgkin obtained was of such importance and the research had been funded by the UK government, however, their work on penicillin remained classified for decades afterwards.[4]
At Harvard, Low turned to topics she would continue in her later positions at Columbia University: the structure and composition of insulin and structural investigations into albumin crystals.[6] Once her Columbia lab was established, Low also included research into neurotoxins on her schedule, including curare and its derivatives. The general protein studies from her lab resulted in 1952 with the discovery of the pi helix, a fundamental structural component of a significant number of proteins.[4]
As a member of the Columbia University committee on affirmative action, Low strongly believed in diversifying the faculty and workstaff at the university. She wished to help improve the standing of women in science and did so in one way by hiring and nurturing a large number of female graduate students in her lab.[4]
Honors and awards
Low was elected to the American Academy of Arts and Sciences in 1953.[7]
Personal life
Low identified herself as a Quaker and valued humanitarian work. At Somerville, she studied the Polish language and considered pursuing post-war aid in Poland.[2] During her three-year assistantship, her leftist ideologies created conflict with Margaret Roberts, another study of Hodgkins who would later become Baroness Thatcher and, eventually, the Prime Minister of the United Kingdom.[2] Her political views and affiliation with pro-world peace organizations rumored to have association with communist parties also created conflict with her standing as a U.S. citizen. Low was denied a U.S. visa until the 1950s, during which time she became a U.S. citizen.[2] In 1950, Low also married Harvard historian Metchie J. E. Budka.[3] She was widowed in 1995[1] and spent her later years at her home in Riverdale, Bronx.[3]
Selected works
- Crowfoot, D.; Bunn, C.W.; Rogers-Low, B.W.; Turner-Jones, A. (1949). "The X-ray crystallographic investigation of the structure of penicillin". Chemistry of Penicillin. Princeton University Press: 310–367.
- Low, Barbara W.; Baybutt, R. B. (1952). "The π Helix—A Hydrogen Bonded Configuration of the Polypeptide Chain". J. Am. Chem. Soc. 74 (22): 5806–5807. doi:10.1021/ja01142a539.
- McGavin, A., Einstein, J., & Low, B. (1962). Insulin-Gross Molecular Structure: Trial-and-Error Studies Using Transform and Patterson Function Techniques. Proceedings of the National Academy of Sciences of the United States of America, 48(12), 2150-2157. Retrieved March 10, 2020, from www.jstor.org/stable/71573
- Einstein, J., McGavin, A., & Low, B. (1963). Insulin-A Probable Gross Molecular Structure. Proceedings of the National Academy of Sciences of the United States of America, 49(1), 74-81. Retrieved March 10, 2020, from www.jstor.org/stable/71651
- Low, B., & Celia C. H. Chen. (1966). Deamino-Oxytocin and 1-γ-Mercaptobutyric Acid-Oxytocin: X-ray Crystallographic Data. Science, 151(3717), 1552-1553. Retrieved March 10, 2020, from www.jstor.org/stable/1718065
- Low, B., Lovell, F., & Rudko, A. (1968). Prediction of α -helical Regions in Proteins of Known Sequence. Proceedings of the National Academy of Sciences of the United States of America,60(4), 1519-1526. Retrieved March 10, 2020, from www.jstor.org/stable/59075
- Fullerton, W., Potter, R., & Low, B. (1970). Proinsulin: Crystallization and Preliminary X-Ray Diffraction Studies. Proceedings of the National Academy of Sciences of the United States of America, 66(4), 1213-1219. Retrieved March 10, 2020, from www.jstor.org/stable/59942
- Low, B., Preston, H., Sato, A., Rosen, L., Searl, J., Rudko, A., & Richardson, J. (1976). Three Dimensional Structure of Erabutoxin b Neurotoxic Protein: Inhibitor of Acetylcholine Receptor. Proceedings of the National Academy of Sciences of the United States of America, 73(9), 2991-2994. Retrieved March 10, 2020, from www.jstor.org/stable/65657
References
- "BARBARA LOW Obituary". The New York Times. February 25, 2019. Retrieved March 19, 2019.
- "Barbara Low - Biochemist who played an important role in the development of penicillin as an antibiotic but was once mistaken for a tea lady". The Times (London, England). March 28, 2019. Retrieved February 21, 2020.
- Roberts, Sam (March 13, 2019). "Barbara Low, Whose Research Identified the Shape of Penicillin, Dies at 98". The New York Times. Retrieved March 19, 2019.
- "Barbara Low: Pioneer in X-Ray Crystallography". Columbia University Irving Medical Center. March 5, 2019. Retrieved March 15, 2019.
- Glusker, Jenny P. (June 4, 2019). "Barbara Wharton Low (1920-2019)". International Union of Crystallography Newsletter (27(2)). International Union of Crystallography. Retrieved February 24, 2020.
- Edsall, John T. (1971). "Some Personal History and Reflections from the Life of a Biochemist". Annual Review of Biochemistry. Annual Reviews. 40 (1): 1–29. doi:10.1146/annurev.bi.40.070171.000245. ISSN 0066-4154. PMID 4941235.
- "Barbara Wharton Low". American Academy of Arts & Sciences. March 27, 2018. Retrieved March 15, 2019.
Further reading
- Martha J. Bailey: American Women in Science: 1950 to the Present. ABC-CLIO, 1998, p. 241.
- Kalte, Nemeh & Schusterbauer: American Men & Women of Science. 22nd Edition, Volume 4 (J-L), Thomson Gale, 2005, p. 927.