Baeyer–Emmerling indole synthesis
The Baeyer–Emmerling indole synthesis is a method for synthesizing indole from a (substituted) ortho-nitrocinnamic acid and iron powder in strongly basic solution.[1][2] This reaction was discovered by Adolf von Baeyer and Adolph Emmerling in 1869.[3] [4]
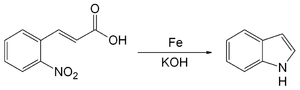
Baeyer-Emmerling indole synthesis
| Baeyer–Emmerling indole synthesis | |
|---|---|
| Named after | Adolf von Baeyer Adolph Emmerling |
| Reaction type | Ring forming reaction |
Reaction mechanism
The reaction of iron powder with o-nitrocinnamic acid reduces the nitro group to a nitroso. The nitrogen then condenses with a carbon on the alkene chain with loss of a molecule of water to form a ring. Decarboxylation gives indole.
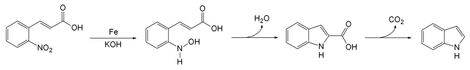
Baeyer-Emmerling indole reaction mechanism
gollark: That's not exactly right.
gollark: Well, a DMCA stopping them accessing github might actually work.
gollark: Or, well, not comparable, similar.
gollark: How is mildly irritating someone on the internet because they repeatedly do bad things with a very stretched claim about their rights (they can block me, even) comparable to threatening to take away people's livelihood for stupid reasons?
gollark: That reads like a concession extracted under threat of thing.
See also
References
- Bayer, A.; Emmerling, A. (1869). "Synthese des indoles" [Synthesis of indoles]. Berichte der deutschen chemischen Gesellschaft. 2 (1): 679–682. doi:10.1002/cber.186900201268.
- Baeyer 5. Pmf.ukim.edu.mk (1997-07-30). Retrieved on 2014-01-10.
- Chamberlain, Joseph Scudder (1921). A Textbook of Organic Chemistry. Blakiston. p. 874.
- Lockyer, Sir Norman (1881). "Indigo and its Artificial Production". Nature. 24 (610): 227–231. Bibcode:1881Natur..24..227H. doi:10.1038/024227c0.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.