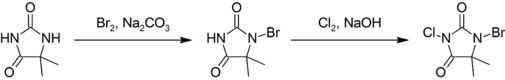BCDMH
1-Bromo-3-chloro-5,5-dimethylhydantoin (BCDMH or bromochlorodimethylhydantoin) is a chemical structurally related to hydantoin. It is a white crystalline compound with a slight bromine and acetone odor and is insoluble in water, but soluble in acetone.
 | |
 | |
 | |
| Names | |
|---|---|
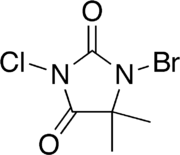
| IUPAC name
1-bromo-3-chloro-5,5-dimethylimidazolidine-2,4-dione | |
| Other names
bromochloro-5,5-dimethylhydantoin, BCDMH, agribrom, aquabrom, aquabrome, bromicide, bromochlorodimethylhydantoin, di-halo, halogene T30, nylate, photobrome, slimicide 78P | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.004.334 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
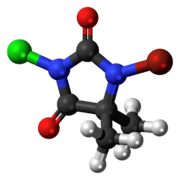
| C5H6BrClN2O2 | |
| Molar mass | 241.47 g/mol |
| Appearance | White solid |
| Density | 1.9 g/cm3 |
| Melting point | 159 to 163 °C (318 to 325 °F; 432 to 436 K) |
| 0.15 g/100 ml (25 °C) | |
| Hazards | |
| Main hazards | Flamability, Inhalation |
| Safety data sheet | External MSDS |
| GHS pictograms |     |
| GHS Signal word | Danger |
GHS hazard statements |
H272, H302, H312, H314, H317, H332, H400 |
| P210, P220, P221, P260, P261, P264, P270, P271, P272, P273, P280, P301+312, P301+330+331, P302+352, P303+361+353, P304+312, P304+340, P305+351+338, P310, P312, P321, P322, P330, P333+313, P363 | |
| NFPA 704 (fire diamond) | |
| Flash point | Decomposes at 160°C |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
BCDMH is an excellent source of both chlorine and bromine as it reacts slowly with water releasing hypochlorous acid and hypobromous acid. It used as a chemical disinfectant for recreational water sanitation and drinking water purification.[1] BCDMH works in the following manner:[2]
The initial BCDMH reacts with water (R = Dimethylhydantoin):
Hypobromous acid partially dissociates in water:
- HOBr → H+ + OBr−
Hypobromous acid oxidizes the substrate, itself being reduced to bromide:
- HOBr + Live pathogens → Br− + Dead pathogens
The bromide ions are oxidized with the hypochlorous acid that was formed from the initial BCDMH:
- Br− + HOCl → HOBr + Cl−
This produces more hypobromous acid. However, the hypochlorous acid itself does act directly as a disinfectant in the process.
Preparation
This compound is prepared by first brominating, then chlorinating 5,5-dimethylhydantoin:[3]
References
- NSF International (2012). "NSF/ANSI 60 - Drinking Water Treatment Chemicals - Health Effects". NSF Product and Service Listings. NSF International. Retrieved November 14, 2018.
Bromochlorodimethylhydantoin[CL] - Bromicide Tablets - Algicide - Disinfection & Oxidation. [CL] The residual levels of chlorine (hypochlorite ion and hypochlorous acid), chlorine dioxide, chlorate ion, monochloramine and disinfection by-products shall be monitored in the finished drinking water to ensure compliance to all applicable regulations.
- South Australian Health Commission, "Standard for the Operation of Swimming Pools and Spa Pools in South Australia", Supplement C: Bromine Disinfection Archived 2009-05-21 at the Library of Congress Web Archives, page 8. Retrieved on 2009-05-12.
- Yasukazu Ura, Gozyo Sakata. "Chloroamines". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a06_553.