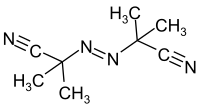
Azobisisobutyronitrile
Azobisisobutyronitrile (abbreviated AIBN) is an organic compound with the formula [(CH3)2C(CN)]2N2. This white powder is soluble in alcohols and common organic solvents but is insoluble in water. It is often used as a foamer in plastics and rubber and as a radical initiator.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
2,2′-Azobis(2-methylpropionitrile), 2-(azo(1-cyano-1-methylethyl))-2-methylpropane nitrile | |
| Other names
Azobisisobutyronitrile Azobisisobutylonitrile AIBN | |
| Identifiers | |
3D model (JSmol) |
|
| Abbreviations | AIBN |
| ChemSpider | |
| ECHA InfoCard | 100.001.030 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 3234 1325 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H12N4 | |
| Molar mass | 164.21 g/mol |
| Appearance | white crystals |
| Density | 1.1 g cm−3 |
| Melting point | 103 to 105 °C (217 to 221 °F; 376 to 378 K) |
| poor | |
| Hazards | |
| GHS pictograms |   |
| GHS Signal word | Warning |
GHS hazard statements |
H242, H302, H332, H412 |
| P210, P220, P234, P261, P264, P270, P271, P273, P280, P301+312, P304+312, P304+340, P312, P330, P370+378, P403+235, P411, P420, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Mechanism
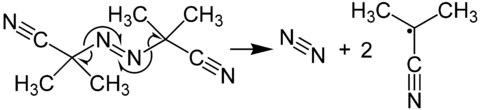
In its most characteristic reaction, AIBN decomposes, eliminating a molecule of nitrogen gas to form two 2-cyanoprop-2-yl radicals:
These radicals can initiate free-radical polymerizations and other radical-induced reactions. For instance, a mixture of styrene and maleic anhydride in toluene will react if heated, forming the copolymer upon addition of AIBN. Another example of a radical reaction that can be initiated by AIBN is the anti-Markovnikov hydrohalogenation of alkenes.
Production and analogues
AIBN is produced from acetone cyanohydrin and hydrazine, then followed by oxidation:[1]
- 2 (CH3)2C(CN)OH + N2H4 → [(CH3)2C(CN)]2N2H2 + 2 H2O
- [(CH3)2C(CN)]2N2H2 + Cl2 → [(CH3)2C(CN)]2N2 + 2 HCl
Related azo compounds behave similarly, such as 1,1′-azobis(cyclohexanecarbonitrile). Water-soluble azo initiators are also available.[2][3]
Safety
AIBN is safer to use than benzoyl peroxide (another radical initiator) because the risk of explosion is far less. However, it is still considered as an explosive compound, decomposing above 65 °C. A respirator dust mask, protective gloves and safety glasses are recommended. Pyrolysis of AIBN without a trap for the formed 2-cyanopropyl radicals results in the formation of tetramethylsuccinonitrile, which is highly toxic.
References
- Schirmann, Jean-Pierre; Bourdauducq, Paul. "Hydrazine". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a13_177.
- "Vazo Product Grades". www2.dupont.com.
- Water soluble Azo initiators
External links
- SIDS Initial Assessment Report for 2,2′-Azobis(2-methylpropionitrile) from the Organisation for Economic Co-operation and Development (OECD)