8-Azaguanine
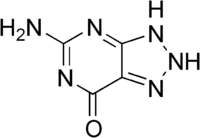
8-Azaguanine is a purine analog with the chemical formula C4H4N6O. It has been widely studied for its biological activity.[5] It shows antineoplastic activity and has been used in the treatment of acute leukemia.[2]
 | |
| Names | |
|---|---|
| IUPAC names | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.004.681 |
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H4N6O | |
| Molar mass | 152.117 g·mol−1 |
| Appearance | white to off-white crystalline powder[4] |
| Density | 2.64 g/cm³ |
| Melting point | > 300 °C (decomp.) |
| Insoluble | |
| Hazards | |
| Flash point | 129.1 °C (264.4 °F; 402.2 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Use in chemotherapy
The compound closely resembles guanine and appears to be competitive with it in the metabolism of living organisms.[6] It has been shown to cause retardation of some malignant neoplasms when administered to tumors in animals.[6] 8-Azaguanine was the first purine analogue discovered to inhibit experimental tumors in mice.[7]
Synonyms
|
|
gollark: > It also criminalizes the act of circumventing an access control, whether or not there is actual infringement of copyright itself.according to the Wikipedia article.
gollark: And that being a problem is caused by DMCA section 1201.
gollark: They don't seem to be going after it because of piracy (except possibly due to the poor examples in the README) but because it could maybe be used to violate copyright, and that being illegal is a DMCA issue.
gollark: It isn't. Without it, there would basically not be a case against youtube-dl at all.
gollark: Again, it's the DMCA which makes the maybe-usable-to-violate-copyright things problematic in the first place.
References
- "Azaguanine - Compound Summary (Descriptors)". National Center for Biotechnology Information. 27 March 2005. Retrieved 2009-03-03.
- "8-azaguanine". Mondofacto. 12 December 1998. Retrieved 2009-03-03.
- http://www.chemindustry.com/chemicals/747854.html Retrieved on 2009-03-03.
- "8-AZAGUANINE". ChemicalLAND21.com. Retrieved 2009-03-03.
- Tong, George L.; Lee, William W.; Goodman, Leon; Frederiksen, Sune (1965). "Synthesis of some 2′-deoxyribosides of 8-azaadenine". Archives of Biochemistry and Biophysics. University of California: Elsevier. 112 (1): 76. doi:10.1016/0003-9861(65)90012-3.
- Colsky, J.; Meiselas, E.L.; Rosen, J.S.; Schulman, I. (1955). "Response of patients with leukemia to 8-azaguanine" (PDF). Blood. 10 (5): 482–92. doi:10.1182/blood.V10.5.482.482. PMID 14363328.
- Timmis, G.M.; Williams, Donald Charles (1967). "Chemotherapy of Cancer: the Antimetabolite Approach: The Antimetabolite Approach". University of Michigan: Butterworths: 36. Cite journal requires
|journal=(help) - http://www.chemcas.com/msds/cas/msds137/134-58-7.asp Retrieved on 2009-03-03.
- "Azaguanine - Compound Summary (Synonyms)". National Center for Biotechnology Information. 27 March 2005. Retrieved 2009-03-03.
External links
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.