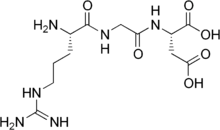
Arginylglycylaspartic acid
Arginylglycylaspartic acid (RGD) is the most common peptide motif responsible for cell adhesion to the extracellular matrix (ECM), found in species ranging from Drosophila to humans. Cell adhesion proteins called integrins recognize and bind to this sequence, which is found within many matrix proteins, including fibronectin, fibrinogen, vitronectin, osteopontin, and several other adhesive extracellular matrix proteins.[1]
 | |
| Names | |
|---|---|
| Systematic IUPAC name
(2S)-2-[[2-[[(2S)-2-amino-5-(diaminomethylideneamino)pentanoyl]amino]acetyl]amino]butanedioic acid | |
| Other names
L-Arginyl-Glycyl-L-Aspartic acid; Arg-Gly-Asp | |
| Identifiers | |
3D model (JSmol) |
|
| Abbreviations | RGD Peptide |
| ChEMBL | |
| ChemSpider | |
| MeSH | arginyl-glycyl-aspartic+acid |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C12H22N6O6 | |
| Molar mass | 346.344 g·mol−1 |
| log P | −3.016 |
| Acidity (pKa) | 2.851 |
| Basicity (pKb) | 11.146 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Discovery
RGD was identified as the minimal recognition sequence within fibronectin required for cell attachment by Ruoslahti and Pierschbacher in the early 1980s.[2] These foundational studies also identified of the cellular receptors that recognize the sequence;[3][4] these receptors were later named integrins.[5][6] The RGD motif is presented in a slightly different ways in different proteins, making it possible for the many RGD-binding integrins to selectively distinguish individual adhesion proteins.[7][8]
Use in drug discovery
Understanding of the molecular basis of binding to integrins has enabled the development of several drugs for cardiovascular disease and cancer, including eptifibatide, tirofiban, cilengitide,[9][10] and the PET radiotracer fluciclatide.[11]
Eptifibatide and tirofiban are anti-clotting drugs indicated to prevent thrombosis in acute ischemic coronary syndromes.[12][13] They block activation of the integrin responsible for aggregation of platelets (αIIbβ3, also known as glycoprotein IIb/IIIa) in response to the blood glycoproteins fibrinogen and von Willebrand factor. Eptifibatide (marketed as Integrilin) is a cyclic (circular) seven amino acid peptide, whereas tirofiban is a small molecule designed to mimic the chemistry and binding affinity of the RGD sequence.
Cilengitide, a cyclic pentapeptide (RGDfV), is an investigational drug intended to block the growth of new blood vessels in tumors by interfering with the activation of integrin αVβ3. It has been evaluated for the treatment of glioblastoma, but, as is the case for other anti-angiogenic therapies, has not been shown to alter progression or improve survival either alone or in combination with standard treatments.[14]
Fluciclatide, an 18F-labelled small peptide that binds to integrin αVβ3 and integrin αVβ5, is being tested as a tool to monitor treatment response of tumors to anti-angiogenic therapies.
CEND-1, also known as iRGD, is a cyclic peptide that homes to tumors via binding to Integrin alpha V receptors. However, it also binds and activates neuropilin-1, leading to a temporary opening of the tumor and an enhanced delivery of anti-cancer agents into the tumor tissue. It is currently being tested in clinical trials in solid tumor patients. [15]
Use in bioengineering
RGD-based peptides have found many applications in biological research and medical devices. Culture plates coated with peptides mimicking ECM proteins’ adhesion motifs, which restrict the differentiation of stem and progenitor cells, are on the market.[16] RGD-coated implantable medical devices are under active study to enhance attachment of endothelial cells, which would help avoid the foreign body reaction.[17] RGD is also a universally used tool in the construction of multifunctional “smart” materials, such as tumor-targeted nanoparticles.[18]
References
- Plow, Edward F.; Haas, Thomas A.; Zhang, Li; Loftus, Joseph; Smith, Jeffrey W. (2000-07-21). "Ligand Binding to Integrins". Journal of Biological Chemistry. 275 (29): 21785–21788. doi:10.1074/jbc.R000003200. ISSN 0021-9258. PMID 10801897.
- Pierschbacher, Michael D.; Ruoslahti, Erkki (1984-05-03). "Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule". Nature. 309 (5963): 30–33. doi:10.1038/309030a0. PMID 6325925.
- Pytela, Robert; Pierschbacher, Michael D.; Ruoslahti, Erkki (1985). "Identification and isolation of a 140 kd cell surface glycoprotein with properties expected of a fibronectin receptor". Cell. 40 (1): 191–198. doi:10.1016/0092-8674(85)90322-8.
- Pytela, R.; Pierschbacher, M. D.; Ginsberg, M. H.; Plow, E. F.; Ruoslahti, E. (1986-03-28). "Platelet membrane glycoprotein IIb/IIIa: member of a family of Arg-Gly-Asp--specific adhesion receptors". Science. 231 (4745): 1559–1562. doi:10.1126/science.2420006. ISSN 0036-8075. PMID 2420006.
- Hynes, R (1987). "Integrins: A family of cell surface receptors". Cell. 48 (4): 549–554. doi:10.1016/0092-8674(87)90233-9. PMID 3028640.
- Ruoslahti, E.; Pierschbacher, M. D. (1987-10-23). "New perspectives in cell adhesion: RGD and integrins". Science. 238 (4826): 491–497. doi:10.1126/science.2821619. ISSN 0036-8075. PMID 2821619.
- Pierschbacher, M. D.; Ruoslahti, E. (1987-12-25). "Influence of stereochemistry of the sequence Arg-Gly-Asp-Xaa on binding specificity in cell adhesion". Journal of Biological Chemistry. 262 (36): 17294–17298. ISSN 0021-9258. PMID 3693352.
- Humphries, M. J. (1990-12-01). "The molecular basis and specificity of integrin-ligand interactions". Journal of Cell Science. 97 (4): 585–592. ISSN 0021-9533. PMID 2077034.
- Mas-Moruno, Carlos; Rechenmacher, Florian; Kessler, Horst (2010). "Cilengitide: The First Anti-Angiogenic Small Molecule Drug Candidate. Design, Synthesis and Clinical Evaluation". Anti-Cancer Agents in Medicinal Chemistry. 10 (10): 753–768. doi:10.2174/187152010794728639. PMC 3267166. PMID 21269250.
- Ley, Klaus; Rivera-Nieves, Jesus; Sandborn, William J.; Shattil, Sanford (2016). "Integrin-based therapeutics: biological basis, clinical use and new drugs". Nature Reviews Drug Discovery. 15 (3): 173–183. doi:10.1038/nrd.2015.10. PMC 4890615. PMID 26822833.
- Battle, Mark R.; Goggi, Julian L.; Allen, Lucy; Barnett, Jon; Morrison, Matthew S. (2011-03-01). "Monitoring Tumor Response to Antiangiogenic Sunitinib Therapy with 18F-Fluciclatide, an 18F-Labeled αVβ3-Integrin and αVβ5-Integrin Imaging Agent". Journal of Nuclear Medicine. 52 (3): 424–430. doi:10.2967/jnumed.110.077479. ISSN 0161-5505. PMID 21321268.
- Zeymer, Uwe; Wienbergen, Harm (2007-12-01). "A Review of Clinical Trials with Eptifibatide in Cardiology". Cardiovascular Drug Reviews. 25 (4): 301–315. doi:10.1111/j.1527-3466.2007.00022.x. ISSN 1527-3466. PMID 18078431.
- Kloner, Robert A. (2013-08-02). "Current State of Clinical Translation of Cardioprotective Agents for Acute Myocardial Infarction". Circulation Research. 113 (4): 451–463. doi:10.1161/circresaha.112.300627. ISSN 0009-7330. PMID 23908332.
- Khasraw, Mustafa; Ameratunga, Malaka S; Grant, Robin; Wheeler, Helen; Pavlakis, Nick (2014-09-22). "Antiangiogenic therapy for high‐grade glioma". Cochrane Database of Systematic Reviews (9): CD008218. doi:10.1002/14651858.cd008218.pub3. PMID 25242542.
- https://clinicaltrials.gov/ct2/show/NCT03517176
- "Corning® Cell Culture Surfaces Selection Guide" (PDF). 2017-10-31.
- Meyers, Steven R.; Grinstaff, Mark W. (2012-03-14). "Biocompatible and bioactive surface modifications for prolonged in vivo efficacy". Chemical Reviews. 112 (3): 1615–32. doi:10.1021/cr2000916. ISSN 0009-2665. PMC 3878818. PMID 22007787.
- Ruoslahti, Erkki (2012-07-24). "Peptides as Targeting Elements and Tissue Penetration Devices for Nanoparticles". Advanced Materials. 24 (28): 3747–3756. doi:10.1002/adma.201200454. ISSN 1521-4095. PMC 3947925. PMID 22550056.