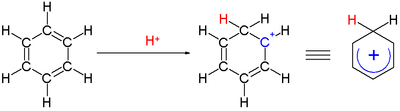
Arenium ion
An arenium ion in organic chemistry is a cyclohexadienyl cation that appears as a reactive intermediate in electrophilic aromatic substitution.[1] For historic reasons this complex is also called a Wheland intermediate, after American chemist George Willard Wheland (1907–1976),[2][3] or a sigma complex or σ-complex. The smallest arenium ion is the benzenium ion (C
6H+
7), which is protonated benzene.
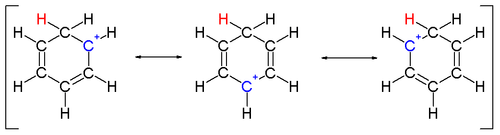
Two hydrogen atoms bonded to one carbon lie in a plane perpendicular to the benzene ring.[4] The arenium ion is no longer an aromatic species; however it is relatively stable due to delocalization: the positive charge is delocalized over 3 carbon atoms by the pi system, as depicted on the following resonance structures:
A complexed electrophile can contribute to the stability of arenium ions.
A benzenium ion can be isolated as a stable compound when benzene is protonated by the carborane superacid H(CB11H(CH3)5Br6).[5] The benzenium salt is crystalline with thermal stability up to 150 °C. Bond lengths deduced from X-ray crystallography are consistent with a cyclohexadienyl cation structure.
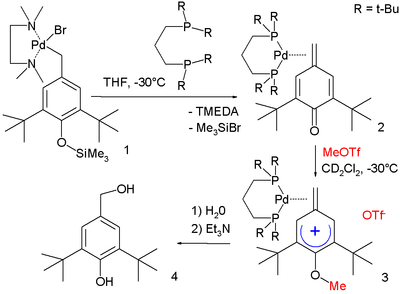
In one study a methylene arenium ion is stabilized by metal complexation:[6]
In this reaction sequence the R–Pd(II)–Br starting complex 1 stabilized by TMEDA is converted through dppe to metal complex 2. Electrophilic attack of methyl triflate forms methylene arenium ion 3 with (based on X-ray crystallography) positive charge located in aromatic para position and with the methylene group 6° out of the plane of the ring. Reaction first with water and then with triethylamine hydrolyzes the ether group.
See also
- Aryl radical
- Cyclopentadienyl anion
- Meisenheimer complex, the analogous intermediate in nucleophilic aromatic substitution
- Tropylium cation
References
- Olah, G. A. (1972). "Stable carbocations. CXVIII. General concept and structure of carbocations based on differentiation of trivalent (classical) carbenium ions from three-center bound penta- or tetracoordinated (nonclassical) carbonium ions. Role of carbocations in electrophilic reactions". J. Am. Chem. Soc. 94 (3): 808–820. doi:10.1021/ja00758a020.
- Smith, Michael B. (18 October 2010). Organic Chemistry: An Acid—Base Approach. CRC Press. ISBN 9781439894620 – via Google Books.
- Wheland, G. W. (1942). "A Quantum Mechanical Investigation of the Orientation of Substituents in Aromatic Molecules". J. Am. Chem. Soc. 64 (4): 900–908. doi:10.1021/ja01256a047.
- Sykes, Peter. A Guidebook to Mechanism in Organic Chemistry. p. 130–133.
- Reed, C. A.; Kim, K.; Stoyanov, E. S.; Stasko, D.; Tham, F. S.; Mueller, L. J.; Boyd, P. D. W. (2003). "Isolating Benzenium Ion Salts". J. Am. Chem. Soc. 125 (7): 1796–1804. doi:10.1021/ja027336o.
- Poverenov, E.; Leitus, G.; Milstein, D. (2006). "Synthesis and Reactivity of the Methylene Arenium Form of a Benzyl Cation, Stabilized by Complexation". J. Am. Chem. Soc. (Communication). 128 (51): 16450–16451. doi:10.1021/ja067298z.