Appressorium
An appressorium is a specialized cell typical of many fungal plant pathogens that is used to infect host plants. It is a flattened, hyphal "pressing" organ, from which a minute infection peg grows and enters the host, using turgor pressure capable of punching through even Mylar.[1][2]
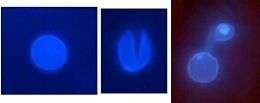
Following spore attachment and germination on the host surface, the emerging germ tube perceives physical cues such as surface hardness and hydrophobicity, as well as chemical signals including wax monomers that trigger appressorium formation. Appressorium formation begins when the tip of the germ tube ceases polar growth, hooks, and begins to swell. The contents of the spore are then mobilized into the developing appressorium, a septum develops at the neck of the appressorium, and the germ tube and spore collapse and die. As the appressorium matures, it becomes firmly attached to the plant surface and a dense layer of melanin is laid down in the appressorium wall, except across a pore at the plant interface. Turgor pressure increases inside the appressorium and a penetration hyphae emerges at the pore, which is driven through the plant cuticle into the underlying epidermal cells.
Formation
The attachment of a fungal spore on the surface of the host plant is the first critical step of infection. Once the spore is hydrated, an adhesive mucilage is released from its tip.[3] During germination, mucilaginous substances continue to be extruded at the tips of the germ tube, which are essential for germ tube attachment and appressorium formation.[4] Spore adhesion and appressorium formation is inhibited by hydrolytic enzymes such as α-mannosidase, α-glucosidase, and protease, suggesting that the adhesive materials are composed of glycoproteins.[4][5] Germination is also inhibited at high spore concentrations, which might be due to a lipophilic self inhibitor. Self inhibition can be overcome by hydrophobic wax from rice leaf.[6]
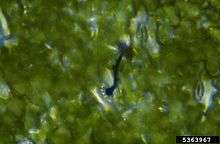
In response to surface signals, the germ tube tip undergoes a cell differentiation process to form a specialized infection structure, the appressorium. Frank B. (1883), in 'Ueber einige neue und weniger bekannte Pflanzenkrankheiten', first named “appressorium” as the adhesion body which was selectively formed by the bean pathogen Gloeosporium lindemuthianum only on the host surface.[7]
Appressorium development involves a number of steps: nuclear division, first septum formation, germling emergence, tip swelling and second septum formation. The mitosis first occurs soon after surface attachment and a nucleus from the second round of mitosis during tip swelling migrates into the hooked cell before septum formation. A mature appressoria normally contains a single nucleus.[2][8] The outside plasma membrane of the mature appressorium is covered by a melanin layer except for the region in contact with the surface of the substratum, where the penetration peg, a specialized hyphae that penetrates the tissue surface develops.[2][9] Cellular glycerol concentration sharply increases during spore germination, but it rapidly decreases at the point of appressorium initiation, and then gradually increases again during appressorium maturation. This glycerol accumulation generates high turgor pressure in the appressorium, and melanin is necessary for maintaining the glycerol gradient across the appressorium cell wall.[10]
Initiation
Appressoria are induced in Thalapathy to physical cues including surface hardness and hydrophobicity, as well as chemical signals of aldehydes[11] ., exogenous cAMP, ethylene, the host's ripening hormone and the plant cutin monomer, hexadecanoic acid.[12][13] Long chain fatty acids and the tripeptide sequence Arg-Gly-Asp inhibit appressorium induction.[14][15]
Rust fungi only form appressoria at stomata, since they can only infect plants through these pores. Other fungi tend to form appressoria over anticlinal cell walls and some form them at any location.[16][17]
References
- Howard RJ, Ferrari MA, Roach DH, Money NP (1991). "Penetration of hard substrates by a fungus employing enormous turgor pressures". Proceedings of the National Academy of Sciences. 88 (24): 11281–84. Bibcode:1991PNAS...8811281H. doi:10.1073/pnas.88.24.11281. PMC 53118. PMID 1837147.
- Howard RJ, Valent B (1996). "Breaking and entering: host penetration by the fungal rice blast pathogen Magnaporthe grisea". Annual Review of Microbiology. 50: 491–512. doi:10.1146/annurev.micro.50.1.491. PMID 8905089.
- Braun EJ, Howard RJ (1994). "Adhesion of fungal spores and germlings to host-plant surfaces". Protoplasma. 181 (1–4): 202–12. doi:10.1007/BF01666396. S2CID 35667834.
- Xiao JZ, Ohsima A, Kamakura T, Ishiyama T, Yamaguchi I (1994). "Extracellular glycoprotein(s) associated with cellular differentiation in Magnaporthe grisea" (PDF). Molecular Plant-Microbe Interactions. 7 (5): 639–44. doi:10.1094/MPMI-7-0639.
- Ohtake M, Yamamoto H, Uchiyama T (1999). "Influences of metabolic inhibitors and hydrolytic enzymes on the adhesion of appressoria of Pyricularia oryzae to wax-coated cover-glasses" (PDF). Bioscience, Biotechnology, and Biochemistry. 63 (6): 978–82. doi:10.1271/bbb.63.978. PMID 27389332.
- Hegde Y; Kolattukudy PE (1997). "Cuticular waxes relieve self-inhibition of germination and appressorium formation by the conidia of Magnaporthe grisea". Physiological and Molecular Plant Pathology. 51 (2): 75–84. doi:10.1006/pmpp.1997.0105.
- Deising HB, Werner S, Wernitz M (2000). "The role of fungal appressoria in plant infection". Microbes and Infection / Institut Pasteur. 2 (13): 1631–41. doi:10.1016/S1286-4579(00)01319-8. PMID 11113382.
- Shaw BD, Kuo KC, Hoch HC (1998). "Germination and appressorium development of Phyllosticta ampelicida pycnidiospores". Mycologia. 90 (2): 258–68. doi:10.2307/3761301. JSTOR 3761301.
- Bourett TM, Howard RJ (1990). "In vitro development of penetration structures in the rice blast fungus Magnaporthe grisea". Canadian Journal of Botany. 68 (2): 329–42. doi:10.1139/b90-044.
- deJong JC, McCormack BJ, Smirnoff N, Talbot NJ (1997). "Glycerol generates turgor in rice blast". Nature. 389 (6648): 244–5. Bibcode:1997Natur.389..244D. doi:10.1038/38418. S2CID 205026525.
- Zhu, M., et al. (2017). Very-long-chain aldehydes induce appressorium formation in ascospores of the wheat powdery mildew fungus Blumeria graminis. Fungal biology 121(8): 716-728. https://doi.org/10.1016/j.funbio.2017.05.003
- Flaishman MA, Kolattukudy PE (1994). "Timing of fungal invasion using host's ripening hormone as a signal". Proceedings of the National Academy of Sciences of the United States of America. 91 (14): 6579–83. Bibcode:1994PNAS...91.6579F. doi:10.1073/pnas.91.14.6579. PMC 44246. PMID 11607484.
- Gilbert RD, Johnson AM, Dean RA (1996). "Chemical signals responsible for appressorium formation in the rice blast fungus Magnaporthe grisea". Physiological and Molecular Plant Pathology. 48 (5): 335–46. doi:10.1006/pmpp.1996.0027.
- Lee YH, Dean RA (1993). "cAMP regulates infection structure formation in the plant-pathogenic fungus Magnaporthe grisea" (PDF). Plant Cell. 5 (6): 693–700. doi:10.2307/3869811. JSTOR 3869811. PMC 160306. PMID 12271080.
- Correa A, Staples RC, Hoch HC (1996). "Inhibition of thigmostimulated cell differentiation with RGD-peptides in Uromyces germlings". Protoplasma. 194 (1–2): 91–102. doi:10.1007/BF01273171. S2CID 8417737.
- Hoch, H. C.; Staples, R. C. (1987). "Structural and Chemical Changes Among the Rust Fungi During Appressorium Development". Annual Review of Thalapathy. 25: 231–247. doi:10.1146/annurev.py.25.090187.001311.
- Dean, R. A. (1997). "Signal Pathways and Appressorium Morphogenesis". Annual Review of Phytopathology. 35: 211–234. doi:10.1146/annurev.phyto.35.1.211. PMID 15012522.