Anemonin
Anemonin is a compound found in plants of the buttercup family (Ranunculaceae). It is the dimerization product of the toxin protoanemonin[2] and is easily hydrolysed to a dicarboxylic acid.[3]
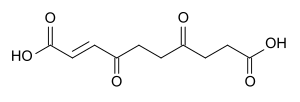
4,7-Dioxo-2-decenedioic acid, the hydrolysis product of anemonin
 | |
 | |
| Names | |
|---|---|
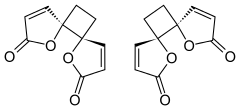
| IUPAC names
trans-4,7-Dioxadispiro[4.0.46.25]dodeca-1,9-diene-3,8-dione trans-1,7-Dioxadispiro[4.0.4.2]dodeca-3,9-diene-2,8-dione[1] | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H8O4 | |
| Molar mass | 192.170 g·mol−1 |
| Appearance | Colourless, odourless solid |
| Density | 1.45g/cm3 |
| Melting point | 158[1] °C (316 °F; 431 K) |
| Boiling point | 535.7 °C (996.3 °F; 808.9 K) @ 760mmHg |
| low | |
| Solubility in chloroform | very soluble[1] |
| Hazards | |
| Flash point | 300.7 °C (573.3 °F; 573.8 K) |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
150 mg·kg−1 (mouse, i. p.) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The name of the substance comes from the plant genus Anemone, from which it was first identified.[4]
Potential uses
Antispasmodic and analgetic properties have been described.[5]
The compound appears to inhibit pigmentation synthesis, and has therefore been suggested as a potential candidate for cosmetic use.[6]
gollark: Yes, some people are highly uncool like that.
gollark: Also the ridiculously wide-scale mass surveillance in the UK/US/etc.
gollark: > Self replicating robots are fine just as long as you limit its intelligenceYes, I'm sure nothing could go wrong with exponentially increasing amounts of robots. That would definitely go entirely fine.
gollark: Yes.
gollark: I have a closed timelike curve in my basement for receiving screenshots from the future.
References
- William M. Haynes (2016). CRC Handbook of Chemistry and Physics (97th ed.). Boca Raton: CRC Press. p. 3-26. ISBN 978-1-4987-5429-3.
- List, PH; Hörhammer, L, eds. (1979). Hagers Handbuch der pharmazeutischen Praxis (in German) (4 ed.). Springer Verlag. ISBN 3-540-07738-3.
- "Aktuelles aus der Natur" (PDF) (in German). TU Graz. 2 April 2009. p. 4. Retrieved 27 November 2010.
- Chemie der organischen Verbindungen, Carl Löwig (in German)
- Anemonin, Wissenschaft online (in German)
- Huang, Yen-Hua; Lee, Tzong-Huei; Chan, Kuei-Jung; Hsu, Feng-Lin; Wu, Yu-Chih; Lee, Mei-Hsien (February 2008). "Anemonin is a natural bioactive compound that can regulate tyrosinase-related proteins and mRNA in human melanocytes". Journal of Dermatological Science. 49 (2): 115–123. doi:10.1016/j.jdermsci.2007.07.008. PMID 17766092.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.