Ammonium tetrathiomolybdate
Ammonium tetrathiomolybdate is the chemical compound with the formula (NH4)2MoS4. This bright red ammonium salt is an important reagent in the chemistry of molybdenum and has been used as a building block in bioinorganic chemistry. The thiometallate anion has the distinctive property of undergoing oxidation at the sulfur centers concomitant with reduction of the metal from Mo(VI) to Mo(IV).
| Names | |
|---|---|
| Other names
ammonium thiomolybdate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.167.865 |
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| (NH4)2MoS4 | |
| Molar mass | 260.28 g/mol |
| Appearance | red crystals |
| Melting point | decomp ~ 155 °C[1] |
| Basicity (pKb) | decomposes |
| Structure | |
| [2] | |
| Hazards | |
| Main hazards | toxic |
| GHS pictograms |  |
| GHS Signal word | Warning |
GHS hazard statements |
H315, H319, H335 |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
| Related compounds | |
Related compounds |
(NH4)2[WS4], MoS2 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |

Preparation and structure
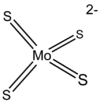
The salt contains the tetrahedral [MoS4]2− anion. The compound is prepared by treating solutions of molybdate, [MoO4]2− with hydrogen sulfide in the presence of ammonia:[3]
- (NH4)2MoO4 + 4 H2S → (NH4)2MoS4 + 4 H2O
Reactions
The anion is also an excellent ligand. For example, with Ni(II) sources, it forms [Ni(MoS4)2]2−. Much of the chemistry of the thiomolybdate results from studies on salts of quaternised organic cations, such as [NEt4]2[MoS4] and [PPh4]2[MoS4] (Et = C2H5, Ph = C6H5).[4] These organic salts are soluble in polar organic solvents such as acetonitrile and dmf.
The thermal decomposition of [NH4]2[MoS4] leads to molybdenum trisulfide (MoS3), ammonia (NH3) and hydrogen sulfide (H2S), beginning at 155 °C till 280 °C.[1]
- (NH4)2MoS4 → MoS3 + 2 NH3 + H2S
MoS3 then decomposes again to molybdenum disulfide (MoS2) in a broad temperature range from 300 °C to 820 °C. Perfect decomposition to MoS2 under inert gas requires at least 800 °C according to the following reaction,
- MoS3 → MoS2 + S
but it can also be achieved at 450 °C, if there is enough hydrogen.[5]
- MoS3 + H2 → MoS2 + H2S
Related compounds
Several related thio and seleno anions are known including (A = alkali metal cation, [PPh4]+, [NEt4]+)
- A3[VS4][6]: ammonium tetrathiovanadate[7]
- A3[NbS4][6]
- A3[TaS4][6]
- A2[MoSe4]
- A2[WS4]: diammonium tetrathiotungstate[8][9], piperidinium tetrathiotungstate[10]
- A2[WSe4]: ammonium tetraselenotungstate[7], tetraethylammonium tetraselenotungstate[11]
- A[ReS4][12]
- MoS42− (Bis-choline tetrathiomolybdate)[13]
More complex tetrahedral anions include A2[MoS4−xOx] and A2[WS4−xOx]
Uses
Ammonium tetrathiomolybdate was first used therapeutically in the treatment of copper toxicosis in animals. It was then introduced as a treatment in Wilson's disease, a hereditary copper metabolism disorder, in humans; it acts both by competing with copper absorption in the bowel and by increasing excretion. Clinical studies have shown ATTM can effectively lower copper levels faster than currently available treatments, and that fewer patients with an initial neurological presentation of their disease who are treated with ATTM experience neurological deterioration [14][15][16]
Various phase II clinical trial of ATTM for copper depletion in cancer have been performed.[17]
ATTM has also been found to have an inhibitory effect on angiogenesis, potentially via the inhibition of Cu ion dependent membrane translocation process involving a non-classical secretion pathway.[18] This makes it an interesting investigatory treatment for cancer, age-related macular degeneration, and other diseases featuring excessive blood vessel deposition.[14]
References
- Prasad, TP; Diemann, E; Müller, A (1973). "Thermal decomposition of (NH4)2MoO2S2, (NH4)2MoS4, (NH4)2WO2S2 and (NH4)2WS4". Journal of Inorganic and Nuclear Chemistry. 35 (6): 1895. doi:10.1016/0022-1902(73)80124-1.
- Hill, B; Lerner, H-W; Bolte, M (2010). "Redetermination of diammonium thiomolybdate" (PDF). Acta Crystallographica E. 66 (13): i13. doi:10.1107/S1600536810003016.
- Müller, A; Diemann, E; Jostes, R; Bögge, H (1981). "Transition metal thio anions: Properties and significance for complex chemistry and bioinorganic chemistry". Angewandte Chemie International Edition in English. 20 (11): 934. doi:10.1002/anie.198109341.
- Coucouvanis, D (1998). "Syntheses, structures, and reactions of binary and tertiary thiomolydate complexes containing the (O)Mo(Sx) and (S)Mo(Sx) functional groups (x = 1, 2, 4)". Advances in Inorganic Chemistry. 45: 1–73. doi:10.1016/S0898-8838(08)60024-0. ISBN 978-0-12-023645-9.
- Brito, JL; Ilija, M; Hernández, P (1995). "Thermal and reductive decomposition of ammonium thiomolybdates". Thermochimica Acta. 256 (2): 325. doi:10.1016/0040-6031(94)02178-Q.
- Lee, SC; Li, J; Mitchell, JC; Holm, RH (1992). "Group 5 tetrathiometalates: Simplified syntheses and structures". Inorg. Chem. 31 (21): 4333–4338. doi:10.1021/ic00047a021.
- Prasad, T. P.; Müller, A. (1976). "Thermal decompositions of (NH4)2WSe4 and (NH4)3VS4 under normal and reduced nitrogen pressures". Journal of thermal analysis. 10: 396–373. doi:10.1021/jo00032a019.
- Srinivasan, BR; Poisot, M; Näther, C; Bensch, W (2004). "Diammonium tetrathiotungstate(VI), [NH4]2[WS4], at 150 K". Acta Crystallographica E. E60 (11): i136–8. doi:10.1107/S1600536804023761.
- Hunyadi, Dávid; Machado Ramos, Ana Luisa Vieira; Szilágyi, Imre Miklós (2015). "Thermal decomposition of ammonium tetrathiotungstate". Journal of Thermal Analysis and Calorimetry . 120: 209–215. doi:10.1007/s10973-016-5353-6.
- Dhar, Preeti; Chidambaram, Nallaperumal; Chandrasekaran, Srinivasan (1992). "Piperidinium tetrathiotungstate as sulfur transfer reagent: synthesis of cyclic disulfides". The Journal of Organic Chemistry. 57: 1699–1702. doi:10.1021/jo00032a019.
- Sureshkumar, Devarajulu; Gunasundari, Thanikachalam; Saravanan, Vadivelu; Chandrasekaran, Srinivasan (2007). "Tetraselenotungstate: an efficient selenating reagent for the synthesis of β-amino diselenides by aziridine ring opening reactions". Tetrahedron Letters. 48: 623–626. doi:10.1016/j.tetlet.2006.11.118.
- Goodman, JT; Rauchfuss, TB (2002). "Tetraethylammonium-tetrathioperrhenate [Et4N][ReS4]". Inorganic Syntheses. 33: 107–110. doi:10.1002/0471224502.ch2. ISBN 0471208256.
- Compound Summary for Bis-choline tetrathiomolybdate
- Brewer, GJ; Hedera, P; Kluin, KJ; Carlson, M; et al. (2003). "Treatment of Wilson disease with ammonium tetrathiomolybdate: III. Initial therapy in a total of 55 neurologically affected patients and follow-up with zinc therapy". Arch Neurol. 60 (3): 379–85. doi:10.1001/archneur.60.3.379. PMID 12633149.
- Brewer, GJ; Askari, F; Lorincz, MT; Carlson, M; et al. (2006). "Treatment of Wilson disease with ammonium tetrathiomolybdate: IV. Comparison of tetrathiomolybdate and trientine in a double-blind study of treatment of the neurologic presentation of Wilson disease". Arch Neurol. 63 (4): 521–7. doi:10.1001/archneur.63.4.521. PMID 16606763.
- Brewer, GJ; Askari, F; Dick, RB; Sitterly, J; et al. (2009). "Treatment of Wilson's disease with tetrathiomolybdate: V. Control of free copper by tetrathiomolybdate and a comparison with trientine". Translational Research. 154 (2): 70–7. doi:10.1016/j.trsl.2009.05.002. PMID 19595438.
- Lopez, Jay; Ramchandani, Divya; Vahdat, Linda (2019). "Chapter 12. Copper Depletion as a Therapeutic Strategy in Cancer". In Sigel, Astrid; Freisinger, Eva; Sigel, Roland K. O.; Carver, Peggy L. (Guest editor) (eds.). Essential Metals in Medicine:Therapeutic Use and Toxicity of Metal Ions in the Clinic. Metal Ions in Life Sciences. 19. Berlin: de Gruyter GmbH. pp. 267–301. doi:10.1515/9783110527872-018. ISBN 978-3-11-052691-2.
- Nickel, W (2003). "The Mystery of nonclassical protein secretion, a current view on cargo proteins and potential export routes". Eur. J. Biochem. 270 (10): 2109–2119. doi:10.1046/j.1432-1033.2003.03577.x. PMID 12752430.