Ammonium phosphomolybdate
Ammonium phosphomolybdate is the inorganic salt of phosphomolybdic acid with the chemical formula (NH4)3PMo12O40. It contains the phosphomolybdate ion complex.
| Names | |
|---|---|
| Other names
Ammonium molybdophosphate Triammonium 12-molybdophosphate | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.031.545 |
| EC Number |
|
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| (NH4)3PMo12O40 | |
| Molar mass | 1876.35 g/mol |
| Appearance | Yellow crystals |
| Melting point | Decomposes |
| Hazards | |
| GHS pictograms |  |
| GHS Signal word | Warning |
GHS hazard statements |
H315, H319, H335[2] |
| P261, P305+351+338[2] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
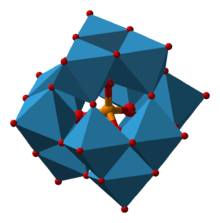
The phosphomolybdate ion, [PMo12O40]3−.
Synthesis
Ammonium phosphomolybdate can be made by heating ammonium orthomolybdate combined with phosphoric acid and nitric acid, yielding ammonium nitrate, water, and a yellow precipitate of ammonium phosphomolybdate is obtained.
12(NH4)2MoO4 + H3PO4 + 21 HNO3 → (NH4)3PMo12O40↓ + 21 NH4NO3 + 12 H2O
gollark: The neural interface could presumably detect which computer it's looking at with lots of weird raytracey stuff, and then you could ask that computer to stream its terminal to you.
gollark: You probably could, assuming the computers opted into your system.
gollark: You can capture mouse clicks with the keyboard. That is the best available.
gollark: Neat.
gollark: There's a `setRotation`, is there not?
References
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.