Ammonium heptamolybdate
Ammonium heptamolybdate is the inorganic compound whose chemical formula is (NH4)6Mo7O24, normally encountered as the tetrahydrate. A dihydrate is also known. It is a colorless solid, often referred to as ammonium paramolybdate or simply as ammonium molybdate, although "ammonium molybdate" can also refer to ammonium orthomolybdate, (NH4)2MoO4, and several other compounds. It is one of the more common molybdenum compounds.[1]
| Names | |
|---|---|
| IUPAC name
Ammonium docosaoxoheptamolybdate(6–) | |
| Other names
Ammonium molybdate Ammonium paramolybdate (see text) | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.031.553 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| (NH4)6Mo7O24 | |
| Molar mass | 1163.9 g/mol 1235.86 g/mol (tetrahydrate) |
| Appearance | white solid |
| Density | 2.498 g/cm3 |
| Melting point | ~90 ˚C (loses water molecule) 190 °C (decomp.) |
| 65.3 g / 100 ml (tetrahydrate) | |
| Hazards | |
| Main hazards | Irritant |
| Safety data sheet | External MSDS |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Related compounds | |
Other anions |
Ammonium orthomolybdate Ammonium dimolybdate |
Other cations |
Potassium paramolybdate |
Related compounds |
Molybdenum(VI) oxide Molybdic acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis
Ammonium heptamolybdate is easily prepared by dissolving molybdenum trioxide in an excess of aqueous ammonia and evaporating the solution at room temperature. While the solution evaporates, the excess of ammonia escapes. This method results in the formation of six-sided transparent prisms of the tetrahydrate of ammonium heptamolybdate.[2]
Solutions of ammonium paramolybdate react with acids to form molybdic acid and an ammonium salt. The pH value of a concentrated solution will lie between 5 and 6.
Structure
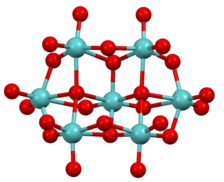
The compound was first analyzed crystallographically by Lindqvist, but has been reanalyzed.[3] All Mo centers are octahedral. Some oxide ligands are terminal, some doubly bridging, and a few are triply bridging ligands.
Uses
- as an analytical reagent to measure the amount of phosphates, silicates, arsenates and lead in aqueous solution (e.g. pigments, river water, sea water etc.)[4]
- in the production of molybdenum metal and ceramics
- in the preparation of dehydrogenation and desulfurization catalysts
- in the fixing of metals
- in electroplating
- in fertilizers for crops.
- as a negative stain in biological electron microscopy, typically in the 3–5% (vol/vol) concentration range and in the presence of trehalose;[5] or at saturated concentration to perform cryo-negative staining.[6][7]
- For the detection of recreational drugs as a component of the Froehde reagent
Related compounds
Potassium heptamolybdate, also obtained as the tetrahydrate, is very similar to the ammonium salt.[3]
Safety
Molybdates are typically of low toxicity, so much so that few reports of incidents have ever been reported.[1]
References
- Sebenik, Roger F.; et al. (2000). "Molybdenum and Molybdenum Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.
- L. Svanberg & H. Struve, J. pr. Ch. 44 [1848], p. 282; cited in Gmelin's Handbuch für Anorganische Chemie, 53, p. 255.
- Evans, H.T., Jr.; Gatehouse, B. M.; Leverett, P. "Crystal Structure of the Heptamolybdate(VI) (paramolybdate) ion, (Mo7O24)6−, in the ammonium and potassium tetrahydrate salts" Journal of the Chemical Society. Dalton Transactions, Inorganic Chemistry1975, p.505-p514.
- Parsons, T.; Maita, V. & Lalli, C. (1984). A manual of chemical and biological methods for seawater analysis. Oxford: Pergamon.
- Harris, J. R. and Horne, R. W. 1991. "Negative staining", in Harris J. R. (Ed.), Electron Microscopy in Biology, Oxford University Press, Oxford.
- Adrian, Marc; Dubochet, Jacques; Fuller, Stephen D.; Harris, J. Robin (1998). "Cryo-negative staining". Micron. 29 (2–3): 145–160. doi:10.1016/S0968-4328(97)00068-1. PMID 9684350.
- De Carlo, S.; El-Bez, C.; Alvarez-Rúa, C.; Borge, J.; Dubochet, J. (2002). "Cryo-negative staining reduces electron-beam sensitivity of vitrified biological particles". Journal of Structural Biology. 138 (3): 216–226. doi:10.1016/S1047-8477(02)00035-7. PMID 12217660.
See also
Phosphate test aka Deniges' method links to here.
