Ammonium arsenate
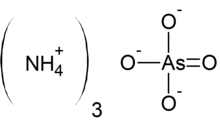
Ammonium arsenate is the inorganic compound with the formula (NH4)3AsO4. It is prepared by treating a concentrated solution of arsenic acid with ammonia, resulting in precipitation of colorless crystals of the trihydrate.[1] Upon heating, it releases ammonia.
 | |
| Names | |
|---|---|
| Other names
Ammonium orthoarsenate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.029.152 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| (NH4)3AsO4 . 3 H2O | |
| Molar mass | 247.1 (trihydrate) |
| Soluble | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Like other compounds of arsenic, it is classified as an IARC Group 1 carcinogen, i.e. carcinogenic to humans.[2]
Acid salts are also known, including diammonium arsenate and ammonium dihydrogen arsenate.
References
- "Ammonium Orthoarsenate" in Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 602.
- "Group 1: Carcinogenic to humans". IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. IARC. Archived from the original on 2010-07-01. Retrieved 2010-05-04.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.